2013 Annual Science Report
 NASA Jet Propulsion Laboratory - Titan
Reporting | SEP 2012 – AUG 2013
NASA Jet Propulsion Laboratory - Titan
Reporting | SEP 2012 – AUG 2013
Task 3.1.1: Stimulated Pre-Biotic Reactions on Titan Surfaces
Project Summary
The program at Georgia Tech. involves Dr. Claire Pirim (postdoctoral researcher) and Dr. Thomas Orlando (PI). It focuses on understanding the reactions occurring on Titan’s surface with an emphasis on determining whether mineral deposits from meteoritic impacts can catalyze the formation of more complex molecules possessing a prebiotic character.
Project Progress
1.) We have completed detailed studies of the thermal and non-thermal processing of pure and mixed formamide ices. Specifically, Fourier transform-infrared spectroscopy (FT-IR) and temperature programmed desorption (TPD) have been used to examine the thermal processing of three isotopes of pure formamide ice (HCONH2, DCONH2, HCOND2) adsorbed on a SiO2 interstellar grain analog. Pure formamide ice on SiO2 nanoparticles displays at least three different phases that we interpret as a porous phase from ~70 145 K, a compacted polycrystalline phase from ~145 210 K, and a third slow diffusion and sublimation phase from ~210 380 K. Water desorbs at 160 K, and formamide has a TPD peak maximum at ~228 K. A mean Eact of ~14.7 kcal/mol (0.64 eV) was obtained using Redhead analysis, indicating strong intermolecular forces within formamide ice. The mixed H2O:HCONH2 ice TPD data suggests possible formamide accumulation if the grains are exposed to temperature cycles < 180 K. In addition, Lyman-α (121.6 nm) photolysis of pure amorphous ice grown at 70 K and crystalline ice produced by annealing to 165 K gives spectral signatures between 2120 and 2195 cm-1 that we assign primarily to OCN¯ and CO. The OCN¯ and CO yields are ~25% less abundant for crystalline ice. Photon and electron processing also produces H2 that is released from the ice between ~90 140 K. Dissociative ionization, direct dissociative excitation and dissociative electron attachment (DEA) channels are accessible in the Lyman-α (121.6 nm) photon and electron-beam energy range studied. DEA energetically favors OCN¯ and H¯ formation, with the latter leading to H2 formation. The fragment product identities, yields and branching ratios are considerably different relative to the gas phase and depend upon the radiation type, ice structure, and the presence of SiO2 nanoparticles. Formation of ions/products is not negligible upon Lyman-α photolysis or electron irradiation, both of which could process ices in Titan’s atmosphere.
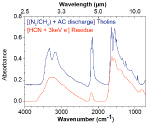
2.) We have examined the role of low energy electrons in the processing of other organic ices such as condensed CH3CN, NH2CH2CN and HCN. DEA resonances were observed between 5-20 eV and the H- desorption cross section from these condensed ices were determined. These experiments show that ices exposed to low-energy electrons may potentially leave heavier neutrals or radicals trapped in the ice. Therefore, while the atmosphere is enriched in H- ions, the ices exposed to low-energy electrons can undergo chemical reactions and charge up. We have therefore also examined the charging behaviour of tholins and some mixed organic ices using electron-stimulated desorption (ESD) experiments and electrostatic force microscopy (EFM). ESD experiments performed at 140K under UHV showed that the tholin film produced by AC discharge (sample provided by Mark Smith) could build a surface potential of about -2eV when exposed to electrons. The surface potential of 2 eV corresponds to a pickup of electrons that are likely trapped due to the presence of the NH3 and CN groups in the tholins, which have large electronegativities. An experiment performed at room temperature using EFM confirmed a surface potential of about 2 eV across a tholin residue produced by the same method. A similar behavior regarding electron pickup was suspected for HCN ice after low-electron energy irradiations. Therefore, a thorough investigation of the changes observed after electron irradiation of condensed HCN at 90K on graphite and SiO2 was done. The results were remarkably similar to those obtained using tholins.
3.) We have developed a chemical mapping technique and protocol using X-ray photoelectron spectroscopy and micro-Raman to evaluate the influence of mineral inclusions on the formation of P O C bonds while thin ices are thermally and radiatively processed. The interest of such chemical mapping experiments has led to the development of spatially resolved surface science techniques in our laboratory.
Publications
-
Bennett, C. J., Pirim, C., & Orlando, T. M. (2013). Space-Weathering of Solar System Bodies: A Laboratory Perspective. Chem. Rev., 113(12), 9086–9150. doi:10.1021/cr400153k
-
Dawley, M. M., Pirim, C., & Orlando, T. M. (2014). Radiation Processing of Formamide and Formamide:Water Ices on Silicate Grain Analogue. The Journal of Physical Chemistry A, 118(7), 1228–1236. doi:10.1021/jp4042815
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Thomas Orlando
Project Investigator
Claire Pirim
Co-Investigator
-
RELATED OBJECTIVES:
Objective 3.1
Sources of prebiotic materials and catalysts
Objective 3.2
Origins and evolution of functional biomolecules