2013 Annual Science Report
 NASA Jet Propulsion Laboratory - Titan
Reporting | SEP 2012 – AUG 2013
NASA Jet Propulsion Laboratory - Titan
Reporting | SEP 2012 – AUG 2013
Task 2.1.1.1: Titan Photochemical Model
Project Summary
To develop a comprehensive model of the chemistry in Titan’s atmosphere including condensation of molecules onto grains, and sublimation back to the gas.
Project Progress
This year’s work has focused on refining and testing the sublimation and condensation code that has been introduced into the KINETICS model. The code is numerically stable and provides a substantial improvement over previous ad hoc methods for treating these processes in Titan’s atmosphere.
There have been computational difficulties in implementing the condensation scheme, which have taken some time to sort out. These have related to the correct treatment of the sublimation rate. The code now correctly replicates the atmospheric mixing ratios expected from consideration of the saturation vapor pressures.
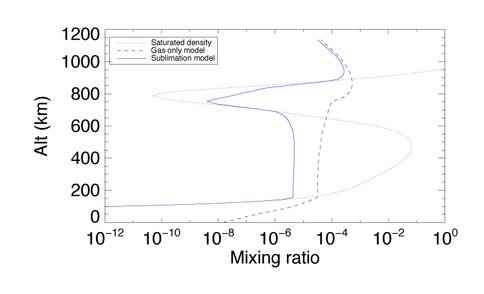
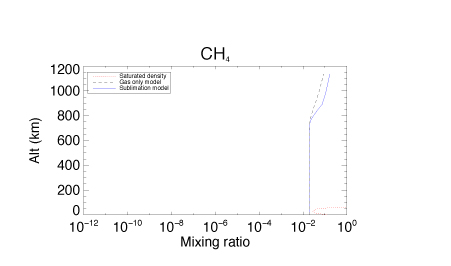
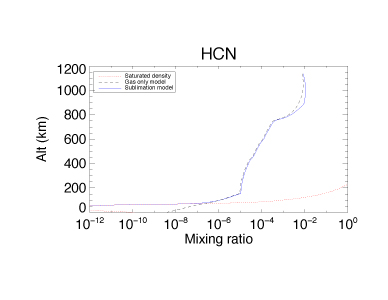
Figures 1 – 3 illustrate how the inclusion of condensation and sublimation processes affect the calculated mixing ratios in Titan’s atmosphere of three molecules: CH4, HCN and HC3N. In the case of CH4 (Figure 1) the mixing ratios are always lower than the saturation densities, resulting in little change in the predictions of models including condensation and sublimation compared to those which consider the gas phase only. For HCN (Figure 2) (and other molecules) the mixing ratios now track the values expected from consideration of the saturation vapor pressure. In the case of HCN, condensation and sublimation result in a lower abundance at altitudes below 50 km. HC3N (Figure 3) shows large changes in mixing ratio compared to the gas only model, and below 700 km the calculated mixing ratios either match or fall well below the expected saturation densities. Above 700 km the inclusion of condensation reduces the mixing ratios markedly over the gas only model, but they still exceed the saturation values. This is because the formation of HC3N in this region is very efficient, and exceeds the rate of condensation of HC3N.
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Karen Willacy
Collaborator
-
RELATED OBJECTIVES:
Objective 2.2
Outer Solar System exploration
Objective 3.1
Sources of prebiotic materials and catalysts