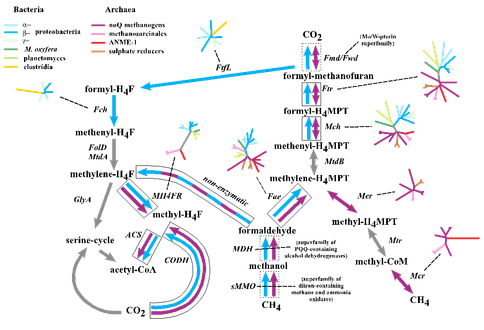2013 Annual Science Report
 NASA Jet Propulsion Laboratory - Icy Worlds
Reporting | SEP 2012 – AUG 2013
NASA Jet Propulsion Laboratory - Icy Worlds
Reporting | SEP 2012 – AUG 2013
Investigation 1: Habitability of Icy Worlds
Project Summary
Habitability of Icy Worlds investigates the habitability of liquid water environments in icy worlds, with a focus on what processes may give rise to life, what processes may sustain life, and what processes may deliver that life to the surface. Habitability of Icy Worlds investigation has three major objectives. Objective 1, Seafloor Processes, explores conditions that might be conducive to originating and supporting life in icy world interiors. Objective 2, Ocean Processes, investigates the formation of prebiotic cell membranes under simulated deep-ocean conditions, and Objective 3, Ice Shell Processes, investigates astrobiological aspects of ice shell evolution.
Project Progress
Investigation 1: Habitability of icy Worlds
Objective 1: Seafloor Processes
This objective investigates conditions that might be conducive to originating and supporting life in icy world interiors. To enable prediction testing by pursuing related questions, we will define biomarker production and look for ground-truth in analog terrestrial systems.
Project 1: Hydrothermal reactor experiments for origin of life Investigators (Co-Is): Michael Russell (lead), Isik Kanik, Steve Vance, Gordon Love, Max Coleman. Members: Laurie Barge, John Baross, Lance Christensen, Richard Kidd, Randall Mielke, and Lauren Spencer-White.
Research: Tests the hypothesis that life can originate through a seafloor process that occurs at temperatures lower than those occurring in hydrothermal systems at mid-ocean ridges.
Year 5 Objectives: Analyze hydrothermal reactor effluents for methane by tunable diode laser spectroscopy (TLS) calibrated and set for both 12CH4 and 13CH4 detection.
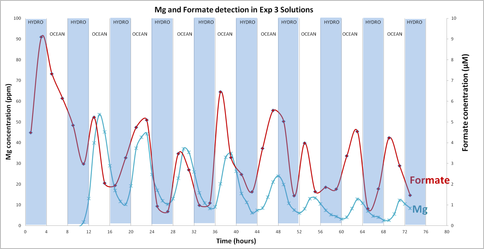
Progress: Although we had previously produced methane in our serpentinizing experiments involving komatiite and pyrrhotite/pentlandite (reactor), its source, whether scrubbed from source rock or reduced from the CO2 feed, was unclear. In experiments conducted with 99.99% enriched 13CO2, measured 13C-enriched methane present was below the detection limit of the instrument (LOD = 1.8 ppb). However, formate (HCOO-) was generated through the partial reduction of CO2 while at the same time Mg was dissolved from synthetic komatiite (Figure 1-1).
Again we failed to produce the thermodynamically challenging formaldehyde (HCHO).
Membrane experiments continued. The partial transformation of mackinawite to greigite (Fe3S4) reached a maximum of 8% of the sulfide at 70°C to 75°C at which temperature lepidocrocite (ɣ-FeOOH) was also produced. Pyrophosphate was also produced these inorganic membranes as analyzed by nuclear magnetic resonance (NMR) (Barge et al., 2012).
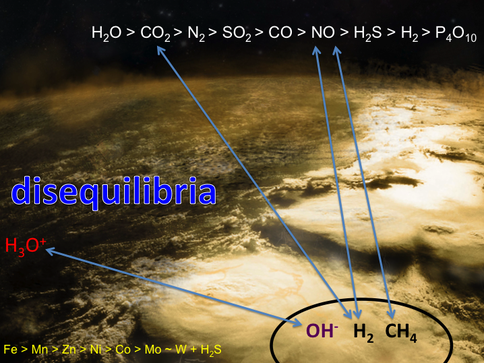
Conclusions: That our attempts to reduce CO2 in our simulations of serpentinization of ultramafic ocean floors stopped at formate and that we failed to go all the way to methane forced a review of our hypothesis favoring the simple reducing acetyl co-A pathway as the gateway to the origin of life. If CO2 could not be easily hydrogenated to CH4 then it seems unlikely that inept early metabolists could make the same transformations. Thus we returned to our earlier ideas that involved disequilibria between for example, high potential electron acceptors such as NO (nitrite and nitrate in water) and the fuels exhaling from alkaline springs (Figure 1-2).
With the phylogenetic contributions of collaborator Wolfgang Nitschke we examined the possibility that the acetyl co-A pathway was involved but driven from both ends rather than just reductively, i.e., the reduction of CO2 to formate and CO as we have partially demonstrated (Figure 1-1), and the concomitant oxidation of methane to methanol, formaldehyde, methylene and a methyl group, and its subsequent condensation to activated acetate. What we have termed the denitrifying methanotrophic acetogenic pathway is a subset of the known methanotrophic pathways involving both the archaea and the bacteria (Figure 1-3a and 1-3b).
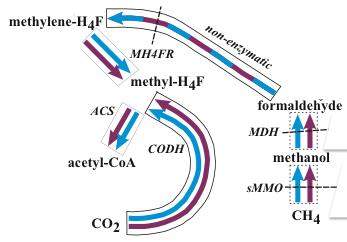
An exciting aspect of this subset is that it involves a minimum of simple enzymes known to be present in the LUCA; di-iron methane monooxygenase, quinol and zinc containing alcohol dehydrogenase, tetrahydrofolate and the nickel iron enzymes, carbon monoxide dehydrogenase and acetyl coenzyme synthase and hydrogenase as detailed in Nitschke et al., 2013 and Schoepp-Cothenetet al., 2013. Nevertheless, because the initial steps to the pathway are endergonic we have given consideration to how these steps might have been driven thermodynamically uphill by redox and pH gradients and have concluded that coupled free energy converting auto-mechanocatalysts must have been coopted from mineralogical ‘objet trouvé’, specifically the green rust comprising part of the membrane (Branscomb and Russell, 2013; Russell et al., 2013).
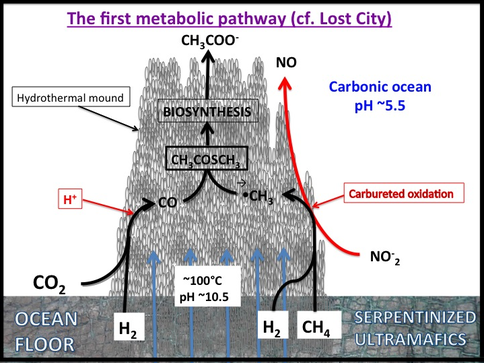
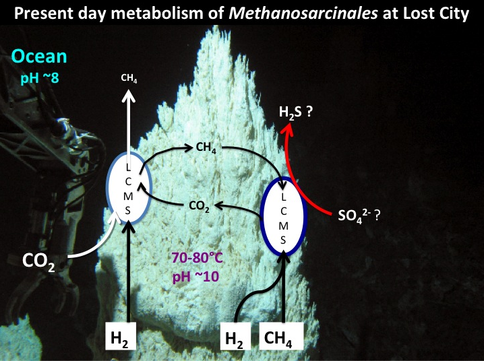
Given that this hypothesis predicted the discovery of the Lost City submarine alkaline hydrothermal vents bearing H2 and CH4 as electron donors and acceptors such as nitrate and sulfate (Figure 1-4), it is noteworthy that the kind of metabolism carried out by Methanosarcinales includes methanotrophy (Figure 1-5). Its reverse, methanogenesis, was a likely a post-LUCA development. This is not to say that Methanosarcinales is primeval, but merely to say that it carries out similar biochemistries to the LUCA (Russell et al., 2013).
Ocean processes:
Co-I’s Vance and Brown published thermodynamic equations of state for magnesium sulfate fluids (vance and Brown, 2013). In this paper they provide the first self-consistent high-precision data suitable for modeling oceans in very large icy satellites. One major finding from their initial application of the results is that salty solutions can have densities higher than those of high pressure ices previously expected to blanket the seafloors of ocean bearing worlds like Ganymede and Titan. This means that, at the 10,000 atmosphere pressures at the bottom of Ganymede’s ocean, or Titan’s, if salty liquids are present, they can participate in water rock interactions of the kind that support life on Earth independent from the sun.
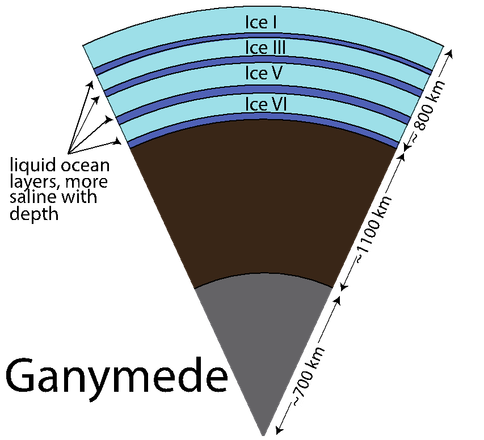
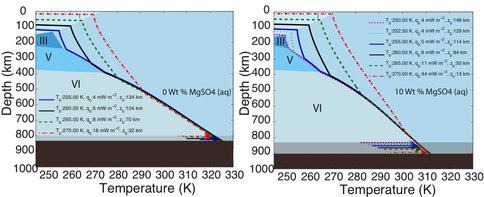
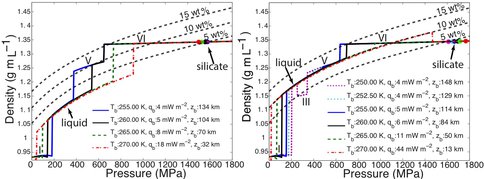
Dr. Vance worked with team members Mathieu Choukroun and Christophe Sotin to develop further application of the high-pressure equations of state. In Summer of 2012 they co-advised a talented Summer Student from France, Mathieu Bouffard, who combined Dr. Choukroun’s previous work on the properties of water ices and melting as a function of pressure and temperature. They used the combined results to predict Ganymede’s internal structure, accounting for the presence of a salty ocean under its icy surface. (Fig. 1-6) They surmise from this work not only that liquids can be remain under high pressure ice phases III, V, and VI, but that for high salinities the are always buoyant, implying that, especially in the case of ice III, snow-like precipitates can form at the seafloor and fall upward. Coupled with the possibility of dense brines periodically being released from Ganymede’s upper ice layer, as happens in sea ice on Earth due to freezing, Ganymede’s ocean begins to look like Earth’s ocean and surface upside down, with the brine outflows replacing hydrothermal systems. All of this makes Ganymede’s ocean seem dynamically interesting, with plenty of energetic processes that can drive chemical disequilbria that might support life. (Figs. 1-7a and 1-7b).
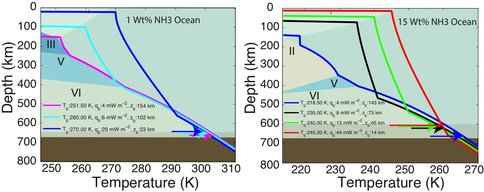
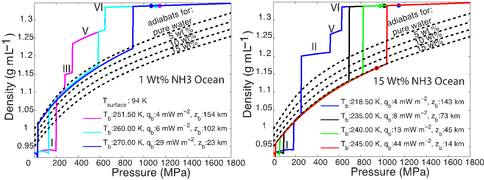
The first paper by Vance et al., 2013 on this topic is under revision with the journal Planetary and Space Science. While visiting the Earth-Life Science Institute at the Tokyo Institute of Technology to work and develop future collaborations, Dr. Vance applied the above methodologies to understanding the role of ammonia in Titan’s ocean Fig. 1-8). Saturn formed far enough away from the Sun that ammonia did not dissociate entirely into molecular nitrogen, which is why materials from the plume of Enceladus contain about 1% ammonia, comparable to a comet. Dr. Vance obtained sound speed measurements in ammonia-water mixtures while working I Dr. Brown’s laboratory in Seattle in 2011. While at ELSI he applied their analytical methods to these results to obtain a new thermodynamic description for the ammonia water system applicable to Titan. The results are self-consistent (e.g., partial derivatives of density with pressure yield accurate sound speeds) and differ substantially from representation for this system created by Croft et al. (1988) in context of Titan.
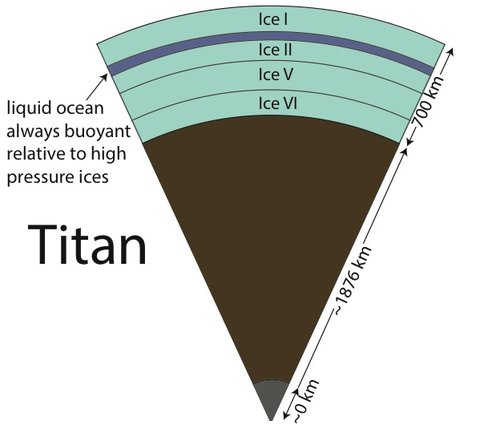
Dr. Vance applied these new results to understanding Titan’s interior structure using a modification of the analytical tools he and the rest of the team developed. To be consistent with Cassini measurements of Titan’s mass and its distribution of density, the results set limits on the depth of Titan’s volatile layer (about 700 km), and do not allow for large iron core, if any. The density of the rocky mantle is low, implying that it should contain a lot of water, meaning that serpentinization is a permissible explanation for Titan’s abundant methane. In contrast with the results for MgSO4, adding ammonia to liquids makes them less dense, so Titan’s high pressure ices would always sink if ammonia were the only thing other than water in its ocean. This is probably not the case, so the intriguing possibility arises that Titan has very salty layers under its high pressure ices, and ammonia concentrates in the upper ocean layer just under the surface. It may even be the case that ammonia stratifies the upper ocean layer so the fluids nearer to the high pressure ice at the ocean’s bottom are less rich in ammonia. Figs. 1-8a and 1-8b)The team is presently writing up these results.
Jason Goodman and Steve Vance have been working to understand fluid and heat transport in icy world oceans, mainly Europa’s. Earlier in the year they submitted a paper applying Vance and Brown’s (2013) magnesium sulfate results to simulating convective flows of salt and heat Europa’s ocean on geological time scales (Vance and Goodman, 2013). The partnership has had roughly equal contribution by the two investigators, with Dr. Goodman writing models based on fluid convection in Earth’s ocean and in the lab, and Dr. Vance running the model to evaluate its implications. They reproduce earlier predictions by Vance and Brown (2005) of the scale salt and heat separation due to diffusive mode double diffusive convection, a phenomenon that could impede biosignatures from reaching Europa’s ice, but also one that might lead to habitable strata at discrete non-convecting boundaries between convecting layers of different salinity and temperature. Vance and Goodman also evaluated the development of a non-convecting layer just under Europa’s ice that results from the anomalous thermal expansion properties of water that are the reason it floats on ice in the first place. The effect also occurs due to melting of the ice if the ocean is salty. In addition to revising this work for publication, Vance and Goodman are developing further studies of ocean dynamics with Prof. Andrew Thompson of Caltech, and Dr. Bruce Bills of JPL.
Dr. Vance worked with a JPL scientist Lance Christensen to develop next generation tunable laser spectrometers for understanding water-rock interactions on Earth and beyond. This year saw tailoring of their technology for future technology to find, characterize, and monitor hydrocarbons in Earth’s ocean, and subsequent development of that technology for future applications in hydrocarbon bearing atmospheres in the solar system. The team demonstrated that tunable laser spectrometers can be developed to simultaneously measure methane, its isotopologues CH3D and 13CH4, well as heavier molecules ethane and propane. These produced in association with each other by biological and geological processes, and are also breakdown products of larger molecules by various chemical and biological mechanisms.
Dr. Vance worked with an international team of scientists at Cabeço de Vide in Portugal in October of 2012 and 2013. Among other findings, they determined that serpentinizing springs at Cabeço de Vide are low in hydrogen relative to similar continental sites on Earth such as The Cedars (Morrill et al. 2013) and Hakuba Happo in Japan (Suda et al. 2013), but contain abundant methane and ethane.
Analyzing gases from fluid samples at Cabeço de Vide has allowed Dr. Vance to perform detailed spectroscopic fitting of the target gases and determine the best approach for characterizing them. This work resulted in a New Technology Report to JPL/Caltech, a precursor to a patent, and Dr. Vance is now pursuing follow-up measurements to demonstrate the concept for use in autonomous underwater vehicles.
These results are important in part because we still do not know how long, how deep underground, or how hot is the serpentinization that occurs. Determining these things can help us figure out how much life Earth’s subsurface might be able to support. With this knowledge we can then apply metrics such as those developed by Vance et al. (2007) to determine how that biopotential scales to smaller worlds with more abundant water and possibly greater depths of fluid-rock interaction.
Vance’s collaborator, José Marques of ICT in Lisbon, has secured funding for the team to visit Portugal in 2014. Dr. Vance is discussing with the team the possibility of inviting researchers from ELSI to also visit the site, and to conduct a comparison visit at a later time to Hakuba Happo in Japan.
Dr. Vance has continued his involvement in project science for the Europa Clipper concept. Icy Worlds Co-I’s Robert Pappalardo, Steve Vance, and Kevin Hand were lead and contributing authors, respectively, on an important paper describing a feasible engineering and scientific design for a Europa lander mission concept (Pappalardo et al., 2013).
References:
S. Vance and J. Brown. Layering and double-diffusion style convection in Europa’s ocean. Icarus, 177(2):506–514, 2005.
P. L. Morrill, J. Gijs Kuenen, O. J. Johnson, S. Suzuki, A. Rietze, A. L. Sessions, M. L. Fogel, and K. H. Nealson. Geochemistry and geobiology of a present-day serpentinization site in California: The Cedars. Geochimica et Cosmochimica Acta, 109:222–240, 2013.
K. Suda, Y. Ueno, M. Yoshizaki, H. Nakamura, K. Kurokawa, E. Nishiyama, K. Yoshino, Y. Hongoh, K. Kawachi, S. Omori, et al. Origin of methane in serpentinite-hosted hydrothermal systems: The CH4– H2–H2O hydrogen isotope systematics of the Hakuba Happo hot spring. Earth and Planetary Science Letters, 386:112–125, 2014.
S. Vance, J. Harnmeijer, J. Kimura, H. Hussmann, B. deMartin, and J. M. Brown. Hydrothermal systems in small ocean planets. Astrobiology, 7(6):987–1005, 2007.
Publications
-
Barge, L. M., Doloboff, I. J., White, L. M., Stucky, G. D., Russell, M. J., & Kanik, I. (2012). Characterization of Iron–Phosphate–Silicate Chemical Garden Structures. Langmuir, 28(8), 3714–3721. doi:10.1021/la203727g
-
Branscomb, E., & Russell, M. J. (2013). Turnstiles and bifurcators: The disequilibrium converting engines that put metabolism on the road. Biochimica et Biophysica Acta (BBA) – Bioenergetics, 1827(2), 62–78. doi:10.1016/j.bbabio.2012.10.003
-
Etiope, G., Vance, S., Christensen, L. E., Marques, J. M., & Costa, I. R. d. (2013). Methane in serpentinized ultramafic rocks in mainland Portugal. Marine and Petroleum Geology, 45, 12–16. doi:10.1016/j.marpetgeo.2013.04.009
-
Nitschke, W., & Russell, M. J. (2013). Beating the acetyl coenzyme A-pathway to the origin of life. Philosophical Transactions of the Royal Society B: Biological Sciences, 368(1622), 20120258–20120258. doi:10.1098/rstb.2012.0258
-
Nitschke, W., McGlynn, S. E., Milner-White, E. J., & Russell, M. J. (2013). On the antiquity of metalloenzymes and their substrates in bioenergetics. Biochimica et Biophysica Acta (BBA) – Bioenergetics, 1827(8-9), 871–881. doi:10.1016/j.bbabio.2013.02.008
-
Pappalardo, R. T., Vance, S., Bagenal, F., Bills, B. G., Blaney, D. L., Blankenship, D. D., … Soderlund, K. M. (2013). Science Potential from a Europa Lander. Astrobiology, 13(8), 740–773. doi:10.1089/ast.2013.1003
-
Russell, M. J., Barge, L. M., Bhartia, R., Bocanegra, D., Bracher, P. J., Branscomb, E., … Kanik, I. (2014). The Drive to Life on Wet and Icy Worlds. Astrobiology, 14(4), 308–343. doi:10.1089/ast.2013.1110
-
Russell, M. J., Nitschke, W., & Branscomb, E. (2013). The inevitable journey to being. Philosophical Transactions of the Royal Society B: Biological Sciences, 368(1622), 20120254–20120254. doi:10.1098/rstb.2012.0254
-
Schoepp-Cothenet, B., Van Lis, R., Atteia, A., Baymann, F., Capowiez, L., Ducluzeau, A-L., … Nitschke, W. (2013). On the universal core of bioenergetics. Biochimica et Biophysica Acta (BBA) – Bioenergetics, 1827(2), 79–93. doi:10.1016/j.bbabio.2012.09.005
-
Vance, S., & Michael Brown, J. (2013). Thermodynamic properties of aqueous MgSO4 to 800MPa at temperatures from −20 to 100°C and concentrations to 2.5molkg−1 from sound speeds, with applications to icy world oceans. Geochimica et Cosmochimica Acta, 110, 176–189. doi:10.1016/j.gca.2013.01.040
-
Vance, S., Bouffard, M., Choukroun, M., & Sotin, C. (2014). Ganymede׳s internal structure including thermodynamics of magnesium sulfate oceans in contact with ice. Planetary and Space Science, 96, 62–70. doi:10.1016/j.pss.2014.03.011
- Vance, S. & Goodman, J. (2013). The influence of salinity on the structure and dynamics of Europa’s ocean and ice shell. Journal Of Geophysical Research-Planets.
- Vance, S., Christensen, L.E. & Aubrey, A. (2013). Carbon Responsive Isotope Reports Laser Spectrometer (CRILS). NTR-49291.
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Steven Vance
Project Investigator
Jason Goodman
Co-Investigator
Isik Kanik
Co-Investigator
Michael Russell
Co-Investigator
Laura Barge
Collaborator
John Baross
Collaborator
Richard Kidd
Collaborator
Randall Mielke
Collaborator
Lauren Spencer-White
Collaborator
-
RELATED OBJECTIVES:
Objective 2.1
Mars exploration.
Objective 2.2
Outer Solar System exploration
Objective 3.2
Origins and evolution of functional biomolecules
Objective 4.1
Earth's early biosphere.
Objective 5.1
Environment-dependent, molecular evolution in microorganisms
Objective 5.2
Co-evolution of microbial communities
Objective 5.3
Biochemical adaptation to extreme environments
Objective 6.2
Adaptation and evolution of life beyond Earth
Objective 7.1
Biosignatures to be sought in Solar System materials
Objective 7.2
Biosignatures to be sought in nearby planetary systems