2013 Annual Science Report
 NASA Goddard Space Flight Center
Reporting | SEP 2012 – AUG 2013
NASA Goddard Space Flight Center
Reporting | SEP 2012 – AUG 2013
Astrobiology in Icy Extraterrestrial Environments
Project Summary
Scientists in the Cosmic Ice Laboratory with the Goddard Center for Astrobiology (GCA) study the formation and stability of molecules under conditions found in outer space. In the past year, studies of amino-acid destruction were continued, a project on the formation of sulfate ions was completed (related to Europa), measurements of the infrared band strengths were published for application to the outer Solar System, and the formation and chemistry of a particularly-versatile interstellar molecule were investigated. All of this work is part of the Comic Ice Laboratory’s continuing contributions to understanding the chemistry of biologically-related molecules and chemical reactions in extra-terrestrial environments.
Project Progress
Our research focuses on chemical reactions and physical properties of icy materials in extraterrestrial environments dominated by low-temperatures (10 – 200 K) and by ionizing radiation. Four projects with astrobiology connections were undertaken in the past year.
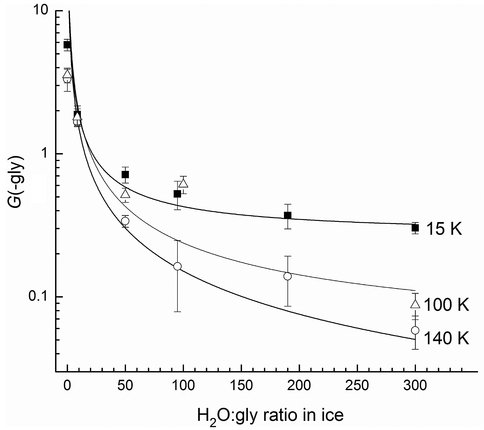
With NAI / GCA support we continued our work on amino acids, focusing strongly on their rates and modes of destruction by radiation on planetary bodies, with a strong emphasis on Mars and its subsurface regions. One specific study revealed that amino acids have a greater rate of radiation resistance (survival) on Mars in higher temperature regions, particularly when diluted in H2O-rich ices (Figure 1). These new measurements lead directly to estimates of the survival rates of glycine and other amino acids on Mars, and extensions to other worlds and other organics are in progress. Surprisingly, almost 40 years after the Viking landers such data from direct in-situ measurements below room temperature are otherwise unavailable.
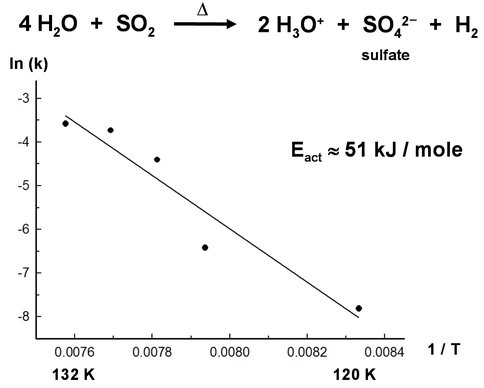
A second project concerned Europa’s icy environment, which contains both strong oxidants (e.g., H2O2) and sulfates. Downward transport of H2O2 through Europa’s surface will bring this molecule into contact with subsurface SO2, forming SO42- for Europan bio-chemistry. Since sulfate-reducing bacteria (e.g., Desulfovibrio vulgaris) are well known, we envision the above reaction as supplying a sulfurous nutrient to an icy extremophile. Our new experiments revealed an efficient reaction for converting SO2 into SO42- (sulfate ion) in low-temperature ices that contain peroxide (H2O2). An Arrhenius plot for the process yields an activation energy for the reaction in H2O-rich ices (Figure 2), which then allows predictions of reaction rates at other temperatures. We are now extending this study to organics and to other strong oxidants, such as perchlorates and ozone.
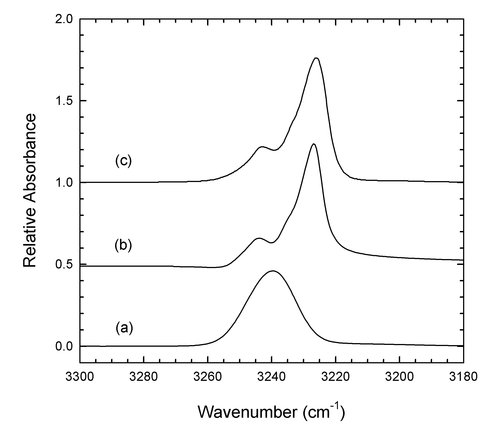
A third project of astrobiological interest concerns the trans-Neptunian region of the Solar System and beyond. To better determine the amount of frozen organics present there we have undertaken an extensive program of spectral quantification, measuring the optical constants of organic compounds. Figure 3 is part of our first published results, on acetylene (C2H2) ices. Traces (a) and (b) are for acetylene’s amorphous and crystalline phases, respectively, near 12 K. Unfortunately, trace© is the literature spectrum of the amorphous phase. Since spectra (a) and (b) of the two phases are strikingly different, any laboratory or astronomical assignments and conclusions based on© will be highly suspect. This situation has been clarified by our recent paper on both ice phases at 12 – 70 K. Work on other organics is in progress.
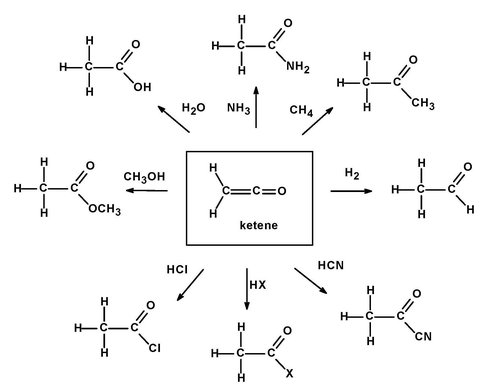
Finally, to better understand extraterrestrial organic chemistry, we investigated the unstable interstellar molecule ketene (H2C=C=O), finding that it is readily made from known cometary and interstellar organics and, of equal interest, leads to predictions of larger organic products (Hudson & Loeffler 2013). Our laboratory work demonstrated, for the first time, that ketene readily forms in both polar and apolar ices starting with known astronomical molecules (e.g., H2O, C2H2). Once made, ketene will generate a rich organic chemistry, as illustrated in Figure 4. We will explore such reactions in the near future.
Publications
-
Gerakines, P. A., & Hudson, R. L. (2013). Glycine’s Radiolytic Destruction in Ices: First in situ Laboratory Measurements for Mars. Astrobiology, 13(7), 647–655. doi:10.1089/ast.2012.0943
-
Hudson, R. L., & Loeffler, M. J. (2013). KETENE FORMATION IN INTERSTELLAR ICES: A LABORATORY STUDY. The Astrophysical Journal, 773(2), 109. doi:10.1088/0004-637x/773/2/109
-
Hudson, R. L., Ferrante, R. F., & Moore, M. H. (2014). Infrared spectra and optical constants of astronomical ices: I. Amorphous and crystalline acetylene. Icarus, 228, 276–287. doi:10.1016/j.icarus.2013.08.029
-
Loeffler, M. J., & Hudson, R. L. (2013). Low-temperature thermal reactions between SO2 and H2O2 and their relevance to the jovian icy satellites. Icarus, 224(1), 257–259. doi:10.1016/j.icarus.2013.02.005
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Reggie Hudson
Project Investigator
Perry Gerakines
Collaborator
Mark Loeffler
Collaborator
-
RELATED OBJECTIVES:
Objective 2.1
Mars exploration.
Objective 2.2
Outer Solar System exploration
Objective 3.1
Sources of prebiotic materials and catalysts
Objective 7.1
Biosignatures to be sought in Solar System materials
Objective 7.2
Biosignatures to be sought in nearby planetary systems