2013 Annual Science Report
 Arizona State University
Reporting | SEP 2012 – AUG 2013
Arizona State University
Reporting | SEP 2012 – AUG 2013
Stoichiometry of Life, Task 2a: Field Studies - Yellowstone National Park
Project Summary
Yellowstone National Park harbors an array of hydrothermal ecosystems with widely varying geochemical characteristics and microbial communities. Our research aims to understand how the geochemistry of these hot springs shapes their constituent microbial communities including their composition and function. To accomplish this aim, we measure (1) physical and geochemical properties of hot spring fluids and sediments, (2) the rates of biogeochemical processes (i.e., methane oxidation, nitrogen fixation, microbial Fe cycling, photosynthesis, de-nitrification, etc.), and (3) markers for microbial community diversity (i.e., SSU rRNA, metabolic genes, lipids, proteins).
Project Progress
Tracking Major Nutrients from Geochemistry to Biochemistry – This area is a major focus that links our fieldwork in Yellowstone with lab analyses and interpretations. We are tracking C and N from hot spring fluids and sediments to biomolecules in microbes including lipids, proteins, and pigments. At the same time we are placing these studies in the broader context of C and N cycles involved in energy conservation and material uptake by microbial communities.
An example of the latter is the documentation by Poret-Peterson et al. (submitted) of methane production and consumption in a small hot spring at the edge of Obsidian Pool by combining stable isotope labeling with gene extraction and enumeration. In particular, we have found an active methane cycle in a slightly acidic hot spring (pH ~6; T=~67oC) using activity assays (tracing the conversion of methane to carbon dioxide) and molecular analyses (detection of transcripts of metabolic genes for methanogenesis and methanotrophy). These results help to quantify how methanotrophic bacteria act as ‘biological filters’ for methane produced by archaea, and emphasize the dynamics of the methane cycle in continental hot springs.
Compound-specific stable isotope measurements were used by Schubotz et al. (2013) to document the functional diversity of seemingly similar chemotrophic microbial communities in alkaline springs of the Lower Geyser Basin. These springs have similar major element compositions including dissolved inorganic carbon, but differing inputs of organic carbon owing to their local settings. High-temperature chemotrophic microbial communities in these springs host microbes that contain similar lipids, but the carbon-isotopic composition of individual lipid classes changes with the availability of dissolved organic carbon, showing distinct evidence of autotrophy in organic-C limited springs to heterotrophy in those receiving organic input from their surroundings. There are major implications in these results for interpreting compound-specific isotopic data from biomarker compounds in the ancient geologic record. This work resulted from a major collaborative effort between Florence Schubotz at MIT and D’Arcy Meyer-Dombard at U. Ill. – Chicago. Prof. Meyer-Dombard has had two students complete master’s degrees over the past year with projects based on element cycling in hot spring ecosystems (Loiacono, 2012; Walther, 2013).
Recent results from lipids extracted from a suite of hot springs by ASU graduate student Grayson Boyer and analyzed by Boyer with Flo Schubotz and Roger Summons at MIT show how molecular compositions of lipids, especially bacterial hopane polyols (BHPs) depend on hot spring compositions suggesting novel uses for the biomarker record. In particular, Boyer has found a strong correlation between the nitrogen content of hot spring fluids and the abundances and varieties of N-bearing BHPs. Results were presented in a poster at the Fall 2013 AGU meeting.
Quantifying the Microbial Iron Cycle – Life in the highest-temperature portions of hot spring ecosystems depends on geochemical sources of energy rather than photosynthesis. In acidic springs the oxidation and reduction of iron are among the ways that microbes gain energy available in geochemical disequilibria. ASU graduate student Brian St. Clair is actively quantifying rates of abiotic and biological iron transformations with field experiments in Yellowstone and elsewhere. His experimental design allows him to quantify reaction rates in killed controls, as well as rates in amended and unamended microcosms. Consistent with other lab studies, St. Clair finds that the abiotic rate of iron oxidation becomes increasingly rapid with increasing pH eventually creating conditions where biological iron oxidation can not compete. Similarly, the abiotic rate of iron reduction increases with decreasing pH, such that microbes are unable to compete with the abiotic rate at extremely acidic conditions. The interplay of these abiotic reaction rates creates a zone of microbial habitability for the iron cycle in hot springs between pH values of roughly 1.5 and 5. In most locations, where there is strong visual evidence of iron oxidation (extensive red ferric oxyhydroxide staining) the rate of microbial iron reduction rivals that of microbial oxidation, implying that the geologic accumulation of iron biominerals may result from a slight shift of the redox cycle balance toward oxidation over time. Results were presented in a talk at the Fall 2013 AGU meeting.
The Transition to Photosynthesis – The transition to photosynthesis is the single most profound change in hot spring ecosystems. On one side the productivities of microbial communities are limited by the amount of geochemical energy provided by disequilibria, while on the other where sunlight is also used as an energy source community productivities are limited by nutrient supplies (Boyd et al., 2012). The multifaceted geochemical and biochemical transitions at the photosynthetic fringe are the focus of coordinated elemental, isotopic, molecular, and modeling studies by Shock and co-workers. The goal is to link the transformations they have begun to document in lipids, proteins, pigments and inorganic compositions along hot spring outflow channels that cross the photosynthetic fringe. Major developments include using metastable equilibrium calculations to characterize the shift in energy supplies. This work builds on thermodynamic calculations pioneered by Dick and Shock (2013), who employed metagenomic data reported by Swingley et al. (2012). ASU post-doctorate student Alysia Cox is extracting proteins from biofilm samples from either side of the photosynthetic fringe guided by metagenomic data from the same samples. At the same time, ASU graduate students Grayson Boyer and Kris Fecteau are extracting lipids and pigments, respectively, from the same samples. This type of integrated study is unprecedented, especially nested in a suite of >70 other geochemical measurements. Among these are determinations of the abundance and chemical speciation of Hg by Fecteau, who is testing whether Hg toxicity suppresses the transition to photosynthesis in some locations. A new proposal to NASA-Exobiology based on preliminary results is pending.
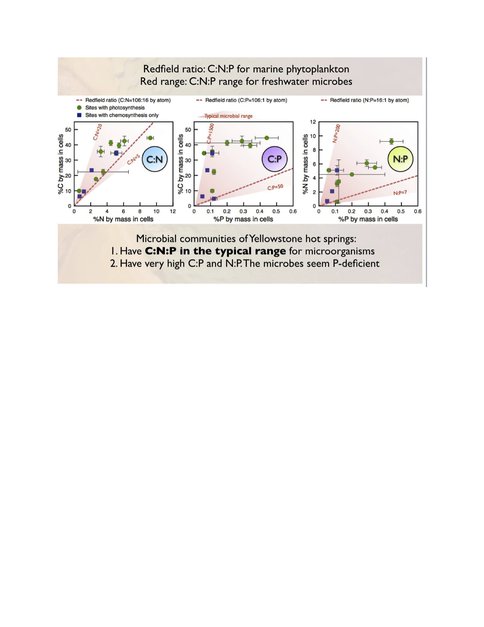
Extended Redfield Ratios: During the 2009 and 2010 field seasons, we collected 200+ sediment and microbial mat samples for determination of the Extended Redfield Ratio (ERR). We measured C, N, P, and trace metal content of bulk sediments and microbial mat samples collected in 2009. We have now isolated cells from this bulk material for ~40 samples using density gradient centrifugation. We have recovered 5% to 40% of the microbial cells from samples and established that their composition is not affected by contamination from the solutions used in the separation procedure (Neveu et al., in submission). Analysis of these cells for C, N, P, and trace metal content yielded C:N:P ratios typical of microbes, even for chemosynthetic communities (Neveu et al., in preparation). Comparison with bulk material elemental compositions showed systematic enrichments in C, N, P, Fe, and ~10 other bio-essential metals in the cells. For some elements (e.g., Mg, Zn), the enrichment patterns were different depending on whether the cells belonged to primarily photosynthetic or chemosynthetic communities. We are also using X-ray microanalysis to measure C, N, P, and S of single cells. Using transmission electron microscopy equipped with an EDX (energy dispersive X-ray spectroscopy) detector (TEM-EDX), measurements on cultured microbes show good accordance between the ratio of C:N obtained via EA-IRMS and by TEM-EDX. We have also measured C, N, P, and S of cells isolated from sediment. Currently, we are testing materials to normalize cellular element measurements to standards of known composition.
MEMS sensor development. We deployed previously developed micro sensors to collect data at Yellowstone National Park and continued efforts to develop new sensor technologies (liquid conductivity sensors) also based on MEMS (Micro-Electrical Mechanical Systems) technology. In the spring of 2012, we set up a long-term temperature monitoring station – with hourly measurements reported via satellite communication from 15 different MEMS temperature sensors in a privately owned hot spring in Gerlach, NV with current continuous measurements spanning one and half year. In the summer of 2013, we deployed arrays of temperature sensors at specifically targeted sites at Yellowstone National Park with transition zones between different microbial communities. In addition we developed arrays of conductivity sensors that were used at YNP in concert with the temperature arrays. This work was presented and published in the preceding of 2013 IEEE Sensors conference.
Publications
-
Boyd, E. S., Fecteau, K. M., Havig, J. R., Shock, E. L., & Peters, J. W. (2012). Modeling the Habitat Range of Phototrophs in Yellowstone National Park: Toward the Development of a Comprehensive Fitness Landscape. Frontiers in Microbiology, 3. doi:10.3389/fmicb.2012.00221
-
Dick, J. M., & Shock, E. L. (2013). A Metastable Equilibrium Model for the Relative Abundances of Microbial Phyla in a Hot Spring. PLoS ONE, 8(9), e72395. doi:10.1371/journal.pone.0072395
-
Oiler, J., Shock, E., Hartnett, H., & Yu, H. (2013). MEMS harsh environment sensor array-enabled hot spring mapping. 2013 IEEE SENSORS. doi:10.1109/icsens.2013.6688332
-
Schubotz, F., Meyer-Dombard, D. R., Bradley, A. S., Fredricks, H. F., Hinrichs, K-U., Shock, E. L., & Summons, R. E. (2013). Spatial and temporal variability of biomarkers and microbial diversity reveal metabolic and community flexibility in Streamer Biofilm Communities in the Lower Geyser Basin, Yellowstone National Park. Geobiology, None, n/a–n/a. doi:10.1111/gbi.12051
-
Swingley, W. D., Meyer-Dombard, D. A. R., Shock, E. L., Alsop, E. B., Falenski, H. D., Havig, J. R., & Raymond, J. (2012). Coordinating Environmental Genomics and Geochemistry Reveals Metabolic Transitions in a Hot Spring Ecosystem. PLoS ONE, 7(6), e38108. doi:10.1371/journal.pone.0038108
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Everett Shock
Project Investigator
Ariel Anbar
Co-Investigator
James Elser
Co-Investigator
Hilairy Hartnett
Co-Investigator
Eric Boyd
Collaborator
Grayson Boyer
Collaborator
Alysia Cox
Collaborator
Jeffrey Dick
Collaborator
Kristopher Fecteau
Collaborator
Kristin Johnson
Collaborator
D'Arcy Meyer-Dombard
Collaborator
Marc Neveu
Collaborator
Jon Oiler
Collaborator
Amisha Poret-Peterson
Collaborator
Florence Schubotz
Collaborator
Brian St Clair
Collaborator
Roger Summons
Collaborator
Hongyu Yu
Collaborator
-
RELATED OBJECTIVES:
Objective 5.1
Environment-dependent, molecular evolution in microorganisms
Objective 5.2
Co-evolution of microbial communities
Objective 5.3
Biochemical adaptation to extreme environments
Objective 6.1
Effects of environmental changes on microbial ecosystems
Objective 6.2
Adaptation and evolution of life beyond Earth
Objective 7.2
Biosignatures to be sought in nearby planetary systems