2013 Annual Science Report
 Arizona State University
Reporting | SEP 2012 – AUG 2013
Arizona State University
Reporting | SEP 2012 – AUG 2013
Habitability of Water-Rich Environments, Task 1: Improve and Test Codes to Model Water-Rock Interactions
Project Summary
Dr. Mikhail Mironenko collaborated with colleagues and completed a code to compute chemical equilibria in low-temperature aqueous systems with salts, CO2 hydrates, and liquid CO2. The code could be used to calculate changes in phase composition during freezing or melting in icy cold environments on Mars, large asteroids, icy moons, comets, and trans-neptunian objects. Dr. C. Glein and Dr. E. Shock have published a model to calculate phase chemical equilibria between several hydrocarbons and N2. The model can be used to explore gas-liquid-solid phase equilibria on Saturn’s moon Titan. Another model has been developed by Dr. Mironenko to calculate condensation of gases in ices and clathrates in the outer solar nebula.
Project Progress
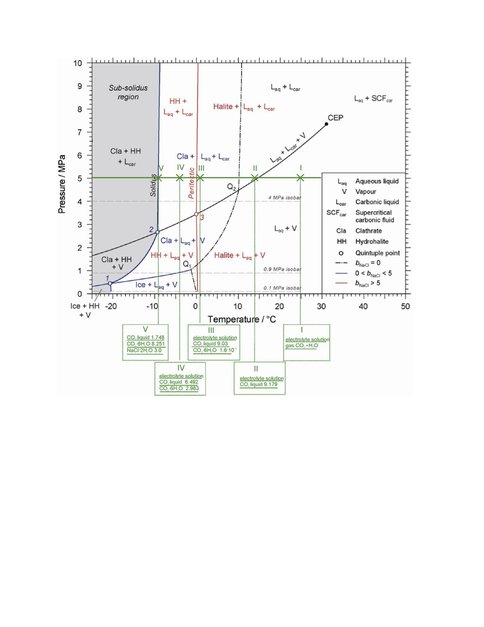
Dr. Mironenko in collaboration with Dr. V. Polyakov and Dr. G. Marion has completed a model for computation of chemical equilibria in CO2-bearing water electrolyte systems. The model includes liquid CO2 and solid hydrate (CO2×6H2O, CO2 clathrate), and incorporates the equation of state for CO2 (Span and Wagner, 1996) into the FREZCHEM database. The authors have incorporated the equation of state for CO2 into algorithms used to calculate phase compositions of multicomponent water-salt-gas type systems. The modeling approach was verified with the phase diagram of Akinfiev and Diamond (2010) for CO2-H2O-NaCl systems (Refer to Figure1). The benefit of the model is the possibility to consider mixed electrolyte solutions, to calculate pH, and take into account hydrolysis of dissolved CO2. The code was tested through calculations of phase composition in heated/cooled isochoric systems via iterative runs of isobaric equilibria. The code could be applied to cold icy and salty aqueous systems with abundant CO2 on Mars, icy moons, and other objects in the outer solar system.
Recent SESE graduate Chris Glein (now at the Carnegie Institution of Washington) and Prof. Everett Shock have published a thermodynamic model to predict phase equilibria between solids, liquids, and gases in the CH4-C2H6-C3H8-C2H2-N2 system at temperatures near the triple point of CH4. The model can be used to explore phase equilibria and surface/atmospheric processes on Saturn’s moon Titan.
Dr. Mironenko had developed a thermodynamic model to calculate condensation of gases to ices and gas hydrates at the conditions of the outer solar system. Published temperature dependencies of equilibrium constants of reactions among gas-hydrates, gases, water ice, and frozen gases have been analyzed and used in a Gibbs free energy minimization code. Condensation of the multicomponent nebular gas into crystalline ice and clathrates has been modeled at conditions of 4-10 astronomical units. This work has been performed in collaboration with Dr. V. Dorofeeva.
Publications
-
Glein, C. R., & Shock, E. L. (2013). A geochemical model of non-ideal solutions in the methane–ethane–propane–nitrogen–acetylene system on Titan. Geochimica et Cosmochimica Acta, 115, 217–240. doi:10.1016/j.gca.2013.03.030
- Dorofeeva, V.A. & Mironenko, M.V. (2012). Conditions of formation of planetary ices. Experiment in Geosciences, 1(1): 15-17.
- Mironenko, M.V., Polyakov, V.B. & Marion, G. (2013). Calculation of equilibria in СО2-bearing water-salt systems. Extension of FREZCHEM mode. Experimental Geochemistry.
- Mironenko, M.V., Polyakov, V.B. & Marion, G.M. (2013). Calculation of equilibria in CO2-bearing water-salt systems. Extension of FREZCHEM model. Experiment in Geosciences, 19(1): 86-89.
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Christopher Glein
Collaborator
Mikhail Mironenko
Collaborator
Venyamin Polyakov
Collaborator
-
RELATED OBJECTIVES:
Objective 2.1
Mars exploration.
Objective 2.2
Outer Solar System exploration