2012 Annual Science Report
 VPL at University of Washington
Reporting | SEP 2011 – AUG 2012
VPL at University of Washington
Reporting | SEP 2011 – AUG 2012
The Long Wavelength Limit for Oxygenic Photosynthesis
Project Summary
Photosynthesis produces signs of life (biosignatures) on a planetary scale: atmospheric oxygen and the reflectance signature of photosynthetic pigments. Oxygenic photosynthesis is therefore a primary target in NASA’s search for life on habitable planets in other solar systems. An unanswered question is what the upper limit is to the photon wavelength at which oxygenic photosynthesis can remain viable. On other planets that have a parent star very different spectrally from our Sun, can we expect oxygen from plants of different colors from those on Earth?
The cyanobacterium, Acaryochloris marina serves as a model organism for oxygenic photosynthesis adapted to low light and red-shifted light environments similar to what may be found on habitable planets orbiting M stars. Until A. marina was discovered in 1996, all known oxygenic photosynthesis relied on the pigment chlorophyll a (Chl a). A. marina instead uses chlorophyll d, which can absorb the far-red and near-infrared light in A. marina’s habitat. We use photoacoustics in the lab to measure the energy storage efficiency of A. marina with lasers, and molecular electrostatics modeling to surmise how replacement of Chl a by Chl d in A. marina affects arrangements within the photosystem molecules. We are finding that A. marina can perform oxygenic photosynthesis quite efficiently in its unique light niche.
Project Progress
Photosynthetic biosignatures — atmospheric oxygen and the spectral reflectance of surface biological pigments — are prime targets in the search for life on extrasolar planets1. Therefore, for this project we have been interested in uncovering rules for the viability of oxygenic photosynthesis and adaptation of photosynthetic pigments to alternative light environments. This research includes lab and modeling studies of the cyanobacterium, Acaryochloris marina, which serves as a model organism for oxygenic photosynthesis adapted to low light and red-shifted light environments similar to what may be found on habitable planets orbiting M stars.
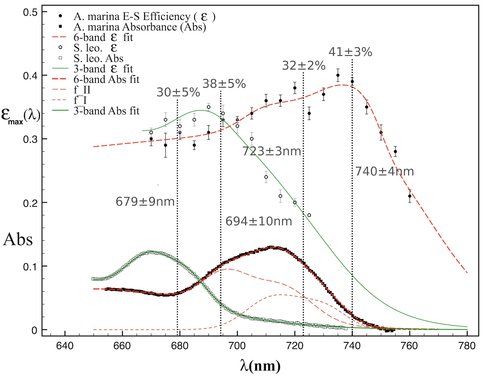
Our lab studies (Mielke, Mauzerall, Blankenship) on A. marina utilize photoacoustics with a tuneable Nd:YAG laser to determine the energy-storage (E-S) efficiency of oxygenic photosynthesis in this chlorophyll (Chl) d-utilizing cyanobacterium2, 3. Our previous work established that A. marina is not thermodynamically limited in its photon energy storage (E- S) efficiency by use of far-red photons (to ≈750 nm). During the reporting period, we significantly extended this work by completing a full spectral characterization of E-S efficiency in A. marina as a function of absorbed wavelength, as well as that in the Chl a cyanobacterium S. leopoliensis. Our results (paper in review3) differentiate contributions to energy-storage by the two photosystems of oxygenic photosynthesis. These provide the first photosystem trap efficiencies and wavelengths obtained directly from energy measurements (Fig. 1). The Blankenship lab has provided verification that the molar ratio of Photosystem I (PSI) to Photosystem II (PSII) in A. marina is near unity, and refined methods for isolating and purifying PSII reaction center complexes.
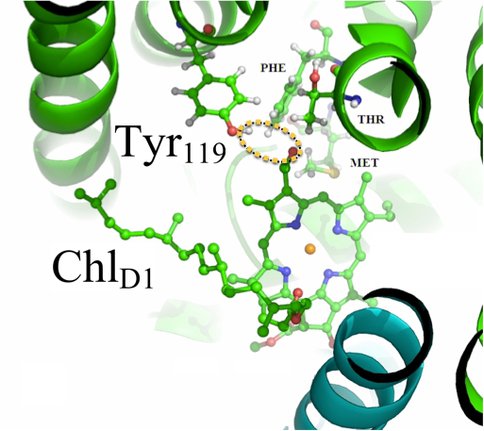
Complementary modeling studies (Mielke, Gunner) consist of molecular modeling and multi-conformation continuum electrostatics (MCCE) calculations to elucidate the impact of replacement of Chl a by Chl d in the reaction centers of A. marina. This past year, we completed redox-potential and binding-energy calculations for electron-transport cofactors in the A. marina PSII reaction center, based on a homology model (Fig. 2). A manuscript is in preparation4. Future research directions will include photoacoustic measurements of the E-S efficiency of purified PSII complexes that will inform and test the partitioning of the contributions of PS I and PSII in the extant in vivo data presented in a paper that is under review 3
Lab culture of the chlorophyll d-utilizing cyanobacterium, Acaryochloris marina
Ideal energy-storage efficiency vs. wavelength, εmax(λ), in whole cells of A. marina (filled circles) and S. leopoliensis (open circles) obtained by photoacoustics. Filled and open squares (lower part of figure) are corresponding sample absorbances. Fitting the data (red and green curves) yields the first trap wavelengths and efficiencies (gray values) obtained directly from energy measurements. The efficiencies in A. marina—32% (PSII) and 41% (PSI)—are slightly higher than those in S. leopoliensis—30% (PSII) and 38% (PSI). Both trap wavelengths are ≈40 nm longer in A. marina—723 vs. 679 nm (PSII) and 740 vs. 694 nm (PSI).
Homology modeling and MCCE calculations show that the mutation Phe119→Tyr119 in the D1 reaction center protein of A. marina favors binding of Chl d at the accessory (ChlD1) site by allowing hydrogen bonding between the Tyr hydroxyl and Chl d formyl moieties (dotted ellipse). Evidence (including that presented in Ref. 3 and Fig. 1) indicates primary charge separation in A. marina occurs at this site.
1. DesMarais, D.J. and L.J.A. Joseph A. Nuth, Alan P. Boss, Jack D. Farmer, Tori M. Hoehler, Bruce M. Jakosky, Victoria S. Meadows, Andrew Pohorille, Bruce Runnegar, Alfred M. Spormann, The NASA Astrobiology Roadmap. Astrobiology, 2008. 8(4): p. 715-730.
2. Mielke, S.P., et al., Efficiency of photosynthesis in a Chl d-utilizing cyanobacterium is comparable to or higher than that in Chl a-utilizing oxygenic species. Biochimica et Biophysica Acta-Bioenergetics, 2011. 1807(9): p. 1231-1236.
3. Mielke, S.P., et al., Photosystem trap energies and spectrally-dependent energy- storage efficiencies in the Chl d-utilizing cyanobacterium, Acaryochloris marina. Biochim. Biophys. Acta . 2012: p. Submitted.
4. Dong, M., S.P. Mielke, and M.R. Gunner, Comparison of chlorophyll a and d electrochemistry and affinity in A. marina and T. elongatus PSII reaction centers. 2012: p. In preparation.
Publications
-
Mielke, S. P., Kiang, N. Y., Blankenship, R. E., & Mauzerall, D. (2013). Photosystem trap energies and spectrally-dependent energy-storage efficiencies in the Chl d-utilizing cyanobacterium, Acaryochloris marina. Biochimica et Biophysica Acta (BBA) – Bioenergetics, 1827(3), 255–265. doi:10.1016/j.bbabio.2012.11.002
-
Mielke, S. P., Kiang, N. Y., Blankenship, R. E., Gunner, M. R., & Mauzerall, D. (2011). Efficiency of photosynthesis in a Chl d-utilizing cyanobacterium is comparable to or higher than that in Chl a-utilizing oxygenic species. Biochimica et Biophysica Acta (BBA) – Bioenergetics, 1807(9), 1231–1236. doi:10.1016/j.bbabio.2011.06.007
-
Nitti, A., Daniels, C. A., Siefert, J., Souza, V., Hollander, D., & Breitbart, M. (2012). Spatially Resolved Genomic, Stable Isotopic, and Lipid Analyses of a Modern Freshwater Microbialite from Cuatro Ciénegas, Mexico. Astrobiology, 12(7), 685–698. doi:10.1089/ast.2011.0812
-
Siefert, J. L., Souza, V., Eguiarte, L., & Olmedo-Alvarez, G. (2012). Microbial Stowaways: Inimitable Survivors or Hopeless Pioneers?. Astrobiology, 12(7), 710–715. doi:10.1089/ast.2012.0833
-
Souza, V., Eguiarte, L. E., Travisano, M., Elser, J. J., Rooks, C., & Siefert, J. L. (2012). Travel, Sex, and Food: What’s Speciation Got to Do with It?. Astrobiology, 12(7), 634–640. doi:10.1089/ast.2011.0768
-
Souza, V., Siefert, J. L., Escalante, A. E., Elser, J. J., & Eguiarte, L. E. (2012). The Cuatro Ciénegas Basin in Coahuila, Mexico: An Astrobiological Precambrian Park. Astrobiology, 12(7), 641–647. doi:10.1089/ast.2011.0675
- Dong, M., Mielke, S.P. & Gunner, M.R. (2012). Comparison of chlorophyll a and d electrochemistry and affinity in A. marina and T. elongatus PSII reaction centers.
- Mauzerall, D. & Mielke., S.P. (in prep.). Energy changes in photosynthetic electron transport: Probing photosynthesis by pulsed photoacoustics. In: Golbeck, J.H. & Van Der Est, A. (Eds.). The Biophysics of Photosynthesis.
- S.P. Mielke, et al. (2011). Efficiency of oxygenic photosynthesis in the far-red light-utilizing cyanobacterium, Acaryochloris marina. Origins 2011: ISSOL and Bioastronomy Joint International Conference. Montpellier, France,.
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
David Mauzerall
Co-Investigator
Robert Blankenship
Collaborator
Marilyn Gunner
Collaborator
-
RELATED OBJECTIVES:
Objective 3.2
Origins and evolution of functional biomolecules
Objective 4.2
Production of complex life.
Objective 5.1
Environment-dependent, molecular evolution in microorganisms
Objective 5.3
Biochemical adaptation to extreme environments
Objective 6.2
Adaptation and evolution of life beyond Earth
Objective 7.2
Biosignatures to be sought in nearby planetary systems