2012 Annual Science Report
 University of Wisconsin
Reporting | SEP 2011 – AUG 2012
University of Wisconsin
Reporting | SEP 2011 – AUG 2012
Project 4E: Preliminary Studies of Fe Isotope Biogeochemistry in Fe-Rich Yellowstone National Park Hot Springs
Project Summary
This preliminary project provided background information for future studies of the structure, function, and signatures (living and non-living) of Fe redox-based microbial life in the volcanic terrain of Yellowstone National Park (YNP). The focus on Fe redox-based systems stems from our expanding knowledge of the wide range of microbial energy metabolisms that are known to be associated with Fe redox transformations on Earth and potentially on other planets. Moreover, Fe redox transformations provide the potential for generation of mineralogical, isotopic, and organic biosignatures of past and present microbial life, which represent premier targets for testing the hypothesis that life currently exists or existed in the past on Mars. Preliminary data on Fe geochemistry and isotopic composition, and microbial community composition, was obtained for two contrasting Fe-rich springs in YNP: Chocolate Pots (CP), a warm, circumneutral environment that has formed on top of the Pleistocene-age Lava Creek Tuff, where a mixture of Fe-rich acid-sulfate geothermal fluids and neutral-pH groundwater from the Gibbon River catchment emerge to the surface; and The Gap site, a hot, acid-sulfate spring in the Norris Basin which supports active chemolithotrophic Fe(II) oxidation, analogous to other hot spring environments in YNP. The geochemical data demonstrated significant changes in aqueous Fe abundance and/or speciation along the flow paths at both sites, leading to accumulation of abundant Fe(III) oxides as well as aqueous Fe(III) at the acidic Gap site. A distinct separation in Fe isotope composition between aqueous Fe and deposited Fe(III) oxides (mainly amorphous Fe-Si coprecipitates) was also detected, with the oxide enriched in 56Fe relative to 54Fe as expected for redox-driven Fe isotope fractionation. However, the degree of fractionation was less the value of ca. 3 ‰ expected in closed system at isotope equilibrium. We suggest that internal regeneration of Fe(II) via dissimilatory Fe(III) reduction could enrich the aqueous Fe(II) pool in the heavy isotope, leading a much lower degree of Fe isotope fractionation – and hence a fundamentally different pattern of Fe isotope fractionation – than would occur in a strictly Fe(II) oxidation-driven reaction system. In support of this argument, an initial set of culturing experiments designed to recovery thermophilic Fe(III)-reducing organisms from CP and Gap materials resulted in the recovery of active Fe(III) reducers from both sites. In addition, preliminary pyrosequencing of 16S rRNA genes recovered from Gap solids provide evidence for Fe(III) reduction potential by the resident microflora. Particularly in the case of the Gap, sequences related to known Archaeal fermenters and elemental S/Fe(III) oxide reducers were abundant.
Project Progress
Recent studies have expanded our understanding of the relationship between volcanism and astrobiology. Cockell and colleagues (2011) conclude that “Given that volcanism is one of the primary mechanisms that generate geochemical disequilibria and fluid migration at a planet’s surface or in its near subsurface, volcanism and life are inextricably linked.” Thus, fluids (e.g. containing reduced compounds such as Fe(II), sulfide, H2, etc.) and mineral solids (e.g. Fe(II)-bearing silicates) produced by hydrothermal processes provide energy substrates that are ideal candidates for the search for ancient life on Earth and possibly on Mars (Jakosky and Shock, 1998).
Understanding the linkage between geochemical (environmental) conditions and the structure, function, and evolution of microbial communities is a central theme in the Astrobiology Roadmap. The genetic record of extant microorganisms documents the outcome of such linkages through Earth history (Banfield and Marshall, 2000; Reysenbach and Shock, 2002). Unraveling the nature of these linkages forms the basis of an emerging area of astrobiology research that seeks to quantify the relationship between the distribution, diversity, and metabolic composition of microbial life and the characteristics of the environment that it inhabits (Cavicchioli, 2002). Recent advances in, geochemistry, molecular biology, and ecological theory enable these relationships to be quantified at a level of resolution that facilitates the prediction of biological data (e.g., genomic composition and diversity) using biogeochemical data (e.g., chemical, physical, isotopic, lipid, etc.) and vice versa (Boyd et al., 2010).
As a component of our ongoing NAI-funded research, we seek to examine in detail the microbiology and geochemistry of two sites in Yellowstone National Park (YNP) that are suitable for dissecting the composition of gene/genomes, extant microbial species, and isotopic, mineralogical, and organic biosignatures in relation to gradients in Fe redox chemistry and other environmental parameters. The two sites we have chosen exhibit contrasting physiochemical properties that encapsulate a range of conditions conducive to the development of Fe-based microbial ecosystems. The Gap (Figure 1A) is a hot (ca. 83 °C at the source), acid-sulfate spring in the Norris Geyser Basin (NGB) which supports active chemolithotrophic Fe(II) oxidation (Kozubal et al., 2012). Chocolate Pots (CP; Figure 1B) is a warm (ca. 52 ° at the source), circumneutral environment that has formed on top of the Pleistocene-age Lava Creek Tuff, where a mixture of Fe-rich acid-sulfate geothermal fluids sourced from the NGB and neutral-pH groundwater sourced from the Gibbon River catchment mix near the surface (McCleskey et al., 2010).
Figure 1. Photos of the Gap (A) and Chocolate Pots (B) sampling sites in YNP. Numbers indicate the sampling locations for the data shown in Figure 2. The photos were taken in August 2011 during a visit by WARC team members to YNP.
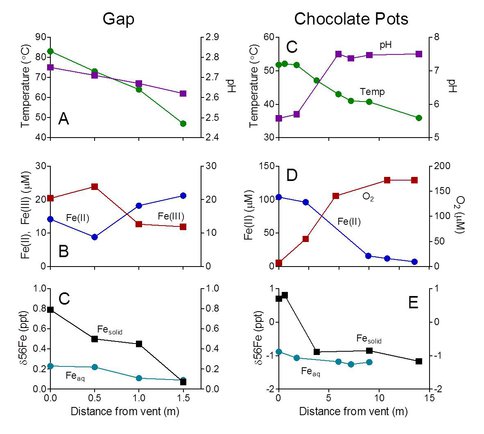
Preliminary data on Fe geochemistry and isotopic composition, and microbial community composition, was obtained for the CP (CP, 2001-2003, Wu et al., 2012, E. Boyd et al., unpubl data) and Gap (E. Roden, unpublished data) hot springs in YNP. The geochemical data (Figure 2) demonstrate significant changes in aqueous Fe abundance and/or speciation along the flow paths at both sites, leading to accumulation of abundant Fe(III) oxides as well as aqueous Fe(III) at the acidic Gap site.
Figure 2. Aqueous geochemical and Fe isotope data for the Gap (A-C; E. Roden et al., unpubl data) and Chocolate Pots (C-E, Wu et al., in prep) sites in YNP.
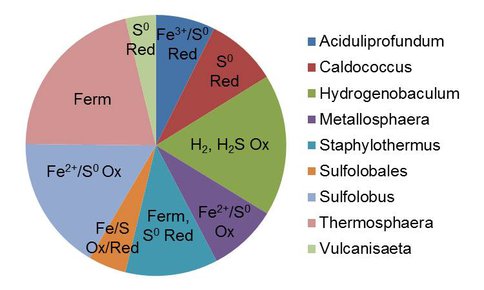
A distinct separation in Fe isotope composition between aqueous Fe and deposited Fe(III) oxides (mainly amorphous Fe-Si coprecipitates) was also detected, with the oxide enriched in 56Fe relative to 54Fe as expected for redox-driven Fe isotope fractionation (Wu et al., 2011). However, the degree of fractionation was less the value of ca. 3 ‰ expected in closed system at isotope equilibrium (Wu et al., 2011). While this degree of fractionation could be attributable to kinetic and equilibrium effects, we suggest that internal regeneration of Fe(II) via dissimilatory Fe(III) reduction could enrich the aqueous Fe(II) pool in the heavy isotope, leading to a much lower degree of Fe isotope fractionation – and hence a fundamentally different pattern of Fe isotope fractionation – than would occur in a strictly Fe(II) oxidation-driven reaction system. In support of this argument, recent enrichment culture studies have directly demonstrated the presence and activity of Fe(III)-reducing organisms in materials from both sites (Roden et al., unpublished data). In addition, preliminary pyrosequence analysis of 16S rRNA genes (ca. 280 bp amplicons of the V4 region, Caporaso et al., 2011) recovered from solids at the two sites (locations 1-4 at the Gap, and location 1 at CP) provide evidence for Fe(II) oxidation by the resident microflora as well as Fe(III) reduction potential. Gap materials in particular show the presence of abundant anaerobic taxa known to be associated with Fe(III) and/or elemental sulfur (S0) reduction, as well as fermentative organisms that presumably generate substrates for these organisms from breakdown of chemolithoautotrophic biomass (Figure 3).
Figure 3. Results of pyrosequencing analysis of 16S rRNA genes in materials from the Gap site. BLAST was used to identify the closest pure culture match (genus level affiliation) to representative operational taxonomic units (OTUs). Pie slice size corresponds to relative OTU abundance out of a combined total of 6276 sequences from the four sampling sites shown in Figure 1. Labels indicate known metabolic capacities for each taxonomic group. Abbreviations: Ferm = Fermentation; Ox = Oxidation; Red = Reduction.
Although sequences related to known Fe(III)-reducing taxa were not abundant in the CP material, this sample was collected from photosynthetic mats at the vent outflow, where anaerobic taxa may not have proliferated due to large inputs of O2 from the atmosphere and from oxygenic photosynthesis (Pierson et al., 1999). We speculate based on these studies and the Fe isotope data that Fe(III) oxide reduction activity takes place further down the flow path, as well as deeper within the deposited materials. The recovery of active Fe(III)-reducing enrichment cultures from non-mat deposits both at the vent outflow and mid-way down the flow path (sites 1 and 6 in Figure 1) is consistent with this idea.
Collectively our preliminary data indicate that the contrasting Gap and CP environments will serve as effective field laboratories for determining how the genomic composition of microbial communities reflects the environmental conditions which support, and observable signatures which record, the presence of Fe-based microbial life. Of particular interest is detection of signatures of the coupling of Fe oxidation and reduction, a key property of modern Fe-based microbial ecosystems that is likely to have applied to early Archean environments on Earth as well as (possibly) Fe-based microbial life on other worlds (Roden and Emerson, 2011; Roden et al., 2012).
Publications
-
Roden, E. E., McBeth, J. M., BlöThe, M., Percak-Dennett, E. M., Fleming, E. J., Holyoke, R. R., … Schieber, J. (2012). The Microbial Ferrous Wheel in a Neutral pH Groundwater Seep. Frontiers in Microbiology, 3. doi:10.3389/fmicb.2012.00172
-
Wu, L., Beard, B. L., Roden, E. E., & Johnson, C. M. (2011). Stable Iron Isotope Fractionation Between Aqueous Fe(II) and Hydrous Ferric Oxide. Environ. Sci. Technol., 45(5), 1847–1852. doi:10.1021/es103171x
-
Wu, L., Brucker, R. P., Beard, B. L., Roden, E. E., & Johnson, C. M. (2013). Iron Isotope Characteristics of Hot Springs at Chocolate Pots, Yellowstone National Park. Astrobiology, 13(11), 1091–1101. doi:10.1089/ast.2013.0996
- Roden, E. & Emerson, D. (2011). Rust never sleeps: a new wave for neutral-pH Fe redox cycling. Environ. Microbiol. Rep, 3(1): 21-23.
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Brian Beard
Co-Investigator
Clark Johnson
Collaborator
Lingling Wu
Postdoc
-
RELATED OBJECTIVES:
Objective 2.1
Mars exploration.
Objective 5.1
Environment-dependent, molecular evolution in microorganisms
Objective 5.3
Biochemical adaptation to extreme environments