2012 Annual Science Report
 University of Wisconsin
Reporting | SEP 2011 – AUG 2012
University of Wisconsin
Reporting | SEP 2011 – AUG 2012
Project 4D: Understanding Conditions of Formation of Mars Evaporite Materials by Quantitative Interpretation of Isotope Compositions of Salts
Project Summary
The variation in the ratios of the naturally occurring (non-radioactive) isotopes of salts dissolved in evaporating water and of the water itself give unparalleled, detailed information on the climate and environment. The existence of hydrated sulfate minerals on Mars and in Terrestrial Martian analog sites indicates that some ancient Martian surface deposits not only precipitated from evaporating surface water but may retain some of that water as part of the crystal structure of the minerals. However, we cannot just analyze the water and use the information because the isotope values are shifted to a small but systematic amount when the water is incorporated in the mineral. We have performed careful laboratory experiments to measure the amount by which the isotope values are shifted for two important mineral types, magnesium sulfate and iron sulfate at a large range of relevant temperatures. In another investigation we have developed a new analytical technique to measure the isotopic compositions of the trace element bromine, similar to the much more common chlorine, present in common salt, sodium chloride. Although these isotopic elements hold a wealth of information progress in using them has been very slow because of the very arduous and lengthily analytical procedures necessary. Our new method allows analysis of samples very much smaller than before and in only a small fraction of the time. These are essential steps in unraveling the climatic and environmental history of Mars.
Project Progress
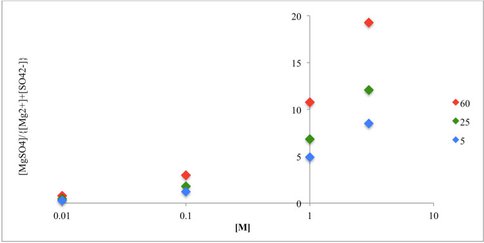
The process of crystallization of salts as a brine evaporates changes the isotopic compositions of hydrogen and oxygen in the water systematically as the process proceeds and also affects other salts in solution. The changes in composition are recorded in successive growth zones in the crystals produced, which can be analyzed subsequently to reveal quantitative details of the evaporation process. In an initial experiment we showed the feasibility of this approach. We have worked on both water of crystallization and a new method for analysis of the halogen elements, chloride and bromide. For the water investigation we have now developed a novel experimental methodology that has enabled us to quantify the isotopic fractionation between free water molecules and those which are bound around an ion in solution, the hydration shell water. However the problem is not simple and data interpretation requires us to differentiate a number of different processes. The proportions of the different ions and un-ionized species in solution varies as a function of concentration as can be seen for the magnesium sulfate system in Figure 1.
Equilibrium thermodynamic model (PHREEQCI) results for general ion formation for solutions of MgSO4 (0.01-2.3 M). Both T and [M] are positively correlated with the abundance of [MgSO4] ion pairs. The effective hydration number increases as [MgSO4] abundance increases.
Despite these complications, we have quantified the fractionation factors (∆18O) for both magnesium and iron sulfates at a range of temperatures from 4°C to 70°C and the results are shown in Figure 2.
Regression lines show 2nd order temperature dependence in most cases and 1st order dependence for the two higher concentrations of magnesium sulfate.
For magnesium sulfate solutions ∆18O decreased with temperature from -6‰ to close to 0‰ between (70-4 °C) in the 2 M solution. Larger temperature effects were observed for solutions of higher concentrations with little to no change observed for 0.5 and 0.25 M solutions. In fact, the lower concentrations show a somewhat expected temperature effect, with ∆18O increasing from 25-4 °C. For ferrous iron sulfate ∆18O showed a stronger temperature effect at 70 °C than for MgSO4, while the differences between 45-4 °C data where smaller. ∆18O maintained a 2nd order relationship with temperature at all concentrations for FeSO4 solutions. At the highest concentration ∆18O decreased from around -8‰ to near -1‰ between 70-4 °C. Again, for the 2 lower concentrations, we observe an inversion point where ∆18O increases in magnitude from 25-4 °C. At 4 °C the concentration effect on ∆18O was smaller than that of MgSO4.
In fact the fractionation factors appear to be similar and the net effect observed results from the change of the ratio of hydration water to free water with more hydration water present at highe temperatures and higher concentrations or molarity (M). The observed inversion point for the low concentration solutions supports the above assertion that hydration number is decreasing with temperature. These solutions are the only ones where the traditionally inverse, temperature vs. isotope fractionation, relationship can be seen. This is because the effective hydration number has decreased enough for the increase in fractionation factor to be observed. At higher concentration and higher temperatures the hydration number (and resulting amount of water captured by a given ion) are more significant than the changes in fractionation factor.
During the evaporation of marine brines, the halide salts (chloride and bromide) being the most soluble species, are the last to be precipitated. During the halite (NaCl) precipitation phase residual bromide accumulates in the brine and changes in the Br/Cl ratio in fluid inclusions can track the evaporation history. However, the amount of material in fluid inclusions is miniscule. Chlorine stable isotope analysis of the halite can give similar quantitative information. It is likely that bromine isotope analysis, complementary to chlorine, will give even more valuable results. Nevertheless, since the first method for such analysis was published 12 years ago, relatively few new results have been produced. Part of the problem has been the extensive and laborious separation of bromide from chloride, followed by its conversion to methyl bromide, the form best suited to analysis in a stable isotope ratio mass spectrometer. In collaboration with colleagues in Paris we have developed a completely novel analytical method in which aqueous bromide is analyzed in an inductively coupled plasma magnetic sector mass spectrometer (Thermo NEPTUNE instrument). As part of this development we developed protocols for separating nanogram quantities of bromide from chloride by ion exchange and also overcame the problem of isotopic interferences from argon compounds formed in the plasma. In fact, to our surprise, the analytical method proved to be unaffected by relatively large amounts of chloride. We have shown that we can analyze nanogram quantities of bromide rapidly to a precision of 0.02‰. The method will be applied first to terrestrial evaporite deposits but very soon also to samples of Martian meteorites. Its most exciting application, analysis of Martian evaporites, will have to wait until such samples are returned to Earth.
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Magali Bonifacie
Co-Investigator
Issaku Kohl
Co-Investigator
Pascale Louvat
Co-Investigator
-
RELATED OBJECTIVES:
Objective 7.1
Biosignatures to be sought in Solar System materials
![Oxygen Isotope Fractionation [Free Water – Hydration Water] for Mg and Fe Sulfate Solutions](../../../../media/site-content/reports/2012/uwis/ae0fe8f65011b4d60aed15050ff9f01a_3522cc96471df7a6b911efa8ea44f3b9_water-of-hydration.jpg.484x0_q85.jpg)
