2012 Annual Science Report
 University of Wisconsin
Reporting | SEP 2011 – AUG 2012
University of Wisconsin
Reporting | SEP 2011 – AUG 2012
Project 4B: Detection of Biosignatures in Extreme Environments, Analogs for Mars
Project Summary
The chemical species sulfate, comprised of sulfur and oxygen atoms, is a constituent of minerals on Earth and on Mars. The small variations in the proportions of the isotopes of oxygen found in sulfate have been used to identify whether and inorganic process or microbiological oxidation of sulfide was responsible for sulfate formation. An intermediate product during the oxidation of sulfide to sulfate is sulfite, which also contains sulfur and oxygen. The work described here shows for the first time the relationship between the oxygen isotope compositions of sulfite and water, which is seen to be a significant control of the oxygen isotope composition of sulfate.
Project Progress
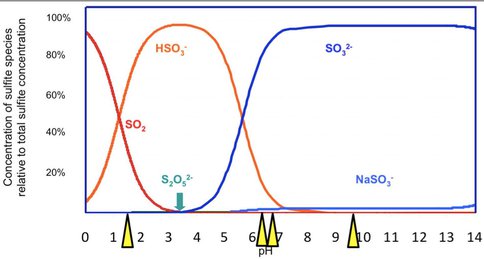
For four years we have been investigating the Río Tinto area, an acid river in Spain analogous to the environment of early Mars. We have shown that the oxygen isotope composition of sulfate produced by microbial oxidation of sulfide characterizes that process. We have now changed the emphasis of the work towards understanding the fundamental controls on the various, relevant isotopic compositions. In collaboration with colleagues at the Max Planck Institute for Marine microbiology in Bremen Germany we have made a major step forward in understanding isotopic compositions of sulfates. The oxygen isotope composition of sulfate in solution and in minerals is very stable and its does not change. However, during the process of its formation, a minor constituent in the reaction pathway, sulfite, can exchange oxygen isotopes with water. There are a number of sulfoxy anion species in chemical equilibrium in solution.
The pH values at which the experiments were conducted are shone by the yellow pointers.
However, there is no agreement on the correct value for the isotopic fractionation between oxygen in sulfite and in water, probably because the experiments are very hard to perform. We used the approach of using a number of different sulfite solutions with different oxygen isotope compositions and precipitating sulfite in the absence of air, which could oxidize it. The experiments were performed at four different pH values, 1.5, 6.3, 6.6, and 9.7, where the proportions of sulfite would be very different from each other. Subsequent analysis determined the equilibrium isotope compositions.
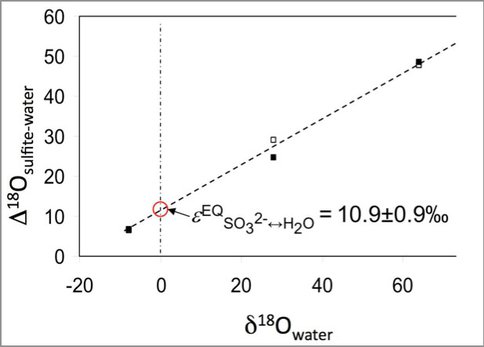
The differences of isotopic compositions of oxygen between sulfite and in the rest of the system for experiments conducted at pH 6.3 and pH6.6 in solutions with different oxygen isotope compositions. Extrapolation to normal water composition gives the isotopic equilibrium fractionation.
Our results provide new insights into the oxygen isotope fractionation during reductive and oxidative sulfur cycling. They demonstrate that isotope exchange between sulfite and water during dissimilatory sulfate reduction (DSR) alone cannot be responsible for the apparent oxygen isotope equilibrium fractionation between sulfate and water mediated by sulfate reducing bacteria. Through these laboratory experiments we have been able to quantify the isotopic fractionation between the relevant sulfoxy anions and water at a range of pH which includes the acid conditions relevant to the Río Tinto. This work will lead to a better understanding of the controls on the compositions of the products of sulfide oxidation.
Publications
-
Müller, I. A., Brunner, B., Breuer, C., Coleman, M., & Bach, W. (2013). The oxygen isotope equilibrium fractionation between sulfite species and water. Geochimica et Cosmochimica Acta, 120, 562–581. doi:10.1016/j.gca.2013.06.037
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Wolfgang Bach
Collaborator
Christian Breuer
Doctoral Student
Inigo Mülle
Doctoral Student
-
RELATED OBJECTIVES:
Objective 7.1
Biosignatures to be sought in Solar System materials