2012 Annual Science Report
 University of Wisconsin
Reporting | SEP 2011 – AUG 2012
University of Wisconsin
Reporting | SEP 2011 – AUG 2012
Project 2E: Preferential Dolomitization of Carbonate Microbialites From a Modern Hypersaline Lake and in Ancient Dolomite
Project Summary
Modern stromatolite from a hypersaline lake displays micro-laminated layers of calcite and protodolomite high-Mg calcite. The oscillatory layers indicate variations of microbial activity. The dolomite layers with micro-crystals are rich in organic. Extracellular polymeric substance (EPS) from photosynthetic microbes enhance precipitation of high-Mg calcite, and EPS from anaerobic bacteria catalyzes the transformation from high-Mg calcite into protodolomite. Atomic resolution Z-contrast images indicate that protodolomite is consisted of very weakly ordered dolomite domains ranging from 3 to 10 nm and disordered dolomite.
Project Progress
Modern stromatolites occur in the Manito Lake in the northern Great Plains in Canada. Manito Lake is a Na-SO4 dominated saline to hypersaline lake. The carbonates mainly occur as pinnacle morphologies with several ten centimeters length. The pinnacles have a core with a sponge like texture and the outer stromatolite-like precipitations in which lighter and darker color submillimeter carbonate layers alternate (Figure 1). Carbonates also precipitate on pebbles and form thin banded layers similar to the outer precipitations of pinnacles. Darker layers are consisting of very fine grains of dolomite or high magnesium calcite, whereas lighter layers are consisting of calcite with several ten microns in size. Organic materials are concentrated in the darker layers, suggesting that organic materials (EPS) worked as catalyst for enhancing dolomite crystallization. Dolomite is so called protodolomite. The composition is deviated from the ideal composition (d104=2.904A ~ 2.933A), and very weak supper lattice reflections are only recognized in the electron diffraction patterns, but not in XRD patterns.
SEM observations revealed that dolomite-rich and calcite-rich layers alternate the same way the darker and lighter layers alternate, but also dolomite-rich and calcite-rich layers cut though each other in some places. The water can penetrate along cracks or porous regions of the stromatolites. The organic materials rich water precipitates dolomites directly and also dolomitize calcites. The change of local environment (such as temperature, evaporation) might be responsible for the alternate texture of dolomite and calcite layers.
X-ray microdiffraction measurements indicate that carbonates do not have supper lattice reflections. They are scalled protodolomite which The d-104 values of carbonates from the banded layers are ranging from 2.933 A to 2.904 A. We examined two samples (d104=2.904A and 2.933A) using a FEI Titan 80-200 aberration corrected scanning/transmission electron microscope operated at 200 kV. Both samples have weak broad super lattice reflections in the electron diffraction patterns. STEM observation revealed that supper lattice domains occur as islands among the disordered dolomite host. Mg-rich dolomite (d104=2.904 A) have supper lattice domains with ~5 nm in size, whereas high Mg calcite (d104=2.933 A) have supper lattice domains with ~3nm in size. A relationship between value and chemical composition is established for the prododolomite.
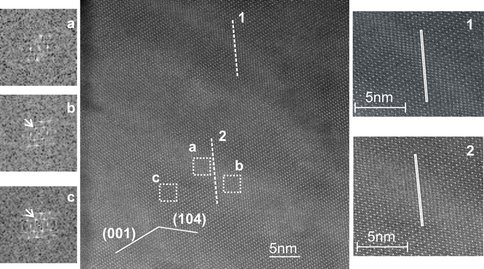
In order to understand Ca-Mg-ordering and exsolution in the Ca-rich dolomite during diagenesis, we iinvestigated an ancient Ca-rich dolomite (Ordovician dolomite from west Wisconsin) using Z-contrast imaging Early stage formed sedimentary dolomite will experience the reaction. Our direct observations of Ca and Mg atoms in the host dolomite and Mg-calcite lamellae indicate that the nano-scale exsolution lamellae are high-Mg calcite with disordered Ca-Mg distribution (Figure 2). Early reported Mg-Ca ordering in the lamellae is due to multiple diffraction resulted from twin-like relationship between the host dolomite and Mg-calcite lamellae.
We also studied Fe-oxides and Fe-hydroxides nano-crystals induced by microbes using transmission electron microscopy and Z-contrast imaging. Part of the results were published in a review article (Konishi et al., 2012).
A stromatolite collected from Monito Lake.
Z-contrast image of a Ca-rich dolomite with Mg-calcite lamellae. 2 lines have been drawn along (1 (-102) to get the compositional variation across the linear feature. Compare the FFTs on the left of the ideal dolomite region and lamellae region. (003) super reflections (in black circles) exists in both FFT patterns.
Publications
-
Konishi, H., & Xu, H. (2012). Nanostructures of Natural Iron Oxide Nanoparticles. Nature’s Nanostructures, None, 75–114. doi:10.1201/b11618-5
-
Shen, Z., Konishi, H., Brown, P. E., & Xu, H. (2013). STEM investigation of exsolution lamellae and “c” reflections in Ca-rich dolomite from the Platteville Formation, western Wisconsin. American Mineralogist, 98(4), 760–766. doi:10.2138/am.2013.4184
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Hiromi Konishi
Research Staff
Zhizhang Shen
Graduate Student
-
RELATED OBJECTIVES:
Objective 7.1
Biosignatures to be sought in Solar System materials
Objective 7.2
Biosignatures to be sought in nearby planetary systems