2012 Annual Science Report
 University of Wisconsin
Reporting | SEP 2011 – AUG 2012
University of Wisconsin
Reporting | SEP 2011 – AUG 2012
Project 2D: Catalytic Role of Adsorbed Hydrogen Sulfide in Dolomite Crystallization: Density Functional Theory Study
Project Summary
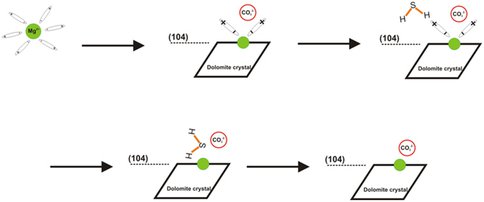
The key kinetic barrier in dolomite crystal formation is believed to be the surface Mg2+-H2O complex which hinder the accessibility of surface Mg2+ ions to the CO32+ ions in the solution. Our recent experiments demonstrated that hydrogen sulfide acting as a catalyst can overcome the barrier and promote dolomite nucleation and growth. Our results from ab initio simulations based on density functional theory (DFT) show that hydrogen sulfide can be adsorbed on (104) surface of dolomite from solution. Aqueous hydrogen sulfide produced by sulfate-reducing bacteria adsorbed on the surface can also increase the surrounding surface Mg2+-H2O distances and thus weaken the electrostatic interactions between surface Mg2+ and water molecules.
Project Progress
The key kinetic barrier in dolomite crystal formation is believed to be the surface Mg2+-H2O complex which hinder the accessibility of surface Mg2+ ions to the CO32- ions in the solution (Lippmann, 1973). It has been demonstrate that dissolved hydrogen sulfide acting as a catalyst can promote dolomite nucleation and growth (Zhang, 2012). It is a consensus that the place where catalysts such as dissolved hydrogen sulfides promote dolomite formation is the surface of dolomite or solid precursor phase (Lippmann, 1973; and Yang et al., 2012). In order to understand the role of dissolved hydrogen sulfide in the dehydration of surface Mg2+ ions, ab initio simulations based on density functional theory (DFT) were carried out to study the thermodynamics of competitive adsorption of hydrogen sulfide and water on dolomite (104) surface from solution. Aqueous hydrogen sulfide among all the species of dissolved hydrogen sulfide was used in this modeling for it is believed to be concentrated on the dolomite surface. According to our simulation results, an aqueous hydrogen sulfide is energetically more strongly bonded to the dolomite surface with adsorption energy -57.26 kJ/mol (in vacuum modeling environment) or -20.77 kJ/mol (in solution modeling environment) lower than that of a water molecule. Thus, aqueous H2S can be more strongly bonded to the dolomite surface than water, thus repulsing the water molecules from the surface.
Since even a small amount of hydrogen sulfide in the solution could promote the Mg2+ incorporation into the calcite structure (Zhang et al., 2012), the effect of adsorbed H2S on the surface magnesium hydration bond is also important to the comprehensive understanding of the role of dissolved hydrogen sulfide. According to our study, the adsorbed aqueous H2S on the surface does not chemically affect the Mg2+-H2O bond but mechanically influences the bond distance. The aqueous hydrogen sulfide adsorbed on the surface can increase the surrounding surface Mg2+-H2O distances and thus weaken the electrostatic interactions between surface Mg2+ and water molecules.
Since dissolved hydrogen sulfides are able to replace the adsorbed water molecules and weaken the surface Mg2+ hydration bond, the question is how carbonate groups manage to be attracted to the surface Mg2+ with the presence of hydrogen sulfides. One speculation is, similar to the H2S effect on Mg2+-H2O bond, that there is room for direct interaction of Mg2+ and CO32- due to the large size and geometry of H2S and larger space between H2S and dolomite surface. It is also possible that the weakened Mg2+-H2O interaction increases the competence of CO32- to bond to the Mg2+. Detailed mechanisms will be explored in our future simulations.
The proposed processes for the incorporation of hydrated Mg2+ to the dolomite surface and the growth of dolomite crystal catalyzed by dissolved hydrogen sulfide.
Publications
-
Zhang, F., Yan, C., Teng, H. H., Roden, E. E., & Xu, H. (2013). In situ AFM observations of Ca–Mg carbonate crystallization catalyzed by dissolved sulfide: Implications for sedimentary dolomite formation. Geochimica et Cosmochimica Acta, 105, 44–55. doi:10.1016/j.gca.2012.11.010
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Izabela Izabela Szlufarska
Collaborator
Fangfu Zhang
Doctoral Student
Zhizhang Shen
Graduate Student
Joshua Kemp
Undergraduate Student
-
RELATED OBJECTIVES:
Objective 7.1
Biosignatures to be sought in Solar System materials
Objective 7.2
Biosignatures to be sought in nearby planetary systems