2012 Annual Science Report
 University of Wisconsin
Reporting | SEP 2011 – AUG 2012
University of Wisconsin
Reporting | SEP 2011 – AUG 2012
Project 2B: The Influence of Temperature, Composition and pCO2 on the Fractionation of Iron Isotopes in the Calcite-Siderite System
Project Summary
While siderite is a relatively common constituent of the sedimentary rock record, little is known about how solution composition and precipitation kinetics affect the trace element and Fe-isotope composition of siderite that forms at temperatures conducive to life (< 100°C). With this knowledge, siderite deposits may be placed within a more meaningful environmental context and the origin and diagenetic history of siderite can be better understood. In this study, inorganic siderite was precipitated under tightly controlled physicochemical conditions using the chemo-stat technique to better understand the factors that influence Fe-isotope fractionation in aqueous systems that contain siderite. Preliminary results suggest that siderite may be grown heterogeneously on pre-existing calcite surfaces but the resulting solids are highly susceptible to oxidation. These factors need to be better understood before inorganic precipitation experiments can be used with confidence to assess factors that influence the Fe-isotope composition of Fe-bearing carbonates.
Project Progress
Siderite occurs in iron-rich sedimentary rocks of all ages, however, little is known about how solution composition (e.g., geochemistry, pH and pCO2) and precipitation kinetics influence the trace element and Fe-isotope composition of siderite that forms at relatively low temperature (< 100°C). With this knowledge, the origin and diagenetic history of ancient siderite deposits may be placed within a more meaningful environmental context.
Most previous laboratory studies have synthesized siderite from metastable precursors under elevated temperatures or pressures (e.g., Carothers et al., 1988; Wersin et al., 1989; Bruno et al., 1992). Singer and Stumm (1970) were the first to precipitate inorganic siderite under surficial conditions (30°C; < 10% CO2), while Dromgoole and Walters (1990) grew CaCO3 inorganically in the presence of aqueous Fe(II) to determine partition coefficients and precipitation kinetics for Fe-calcite. More recently, Jiménez-López and Romanek (2004) precipitated pure siderite at 25°C using the chemo-stat technique. To date, no experiment has characterized the effect of aqueous Ca or Mg on the formation of siderite at low temperature nor has any study characterized the effect of temperature, precipitation kinetics or solution chemistry on the fractionation of Fe-isotopes in calcite-siderite solid solutions.
To fill this data gap, a series of chemo-stat experiments was conducted to grow siderite heterogeneously on calcite seed material under tightly controlled physicochemical conditions. Unlike the chemo-stat experiments described in Task 2A, additional technical hurdles had to be overcome to acquire meaningful experimental results. First, all solutions must be prepared in an oxygen free environment. Second, gaseous mass flow/transport and gas-fluid equilibria considerations require all CO2 and CO2/N2 gas mixtures to be purified over hot Cu metal to remove trace quantities of oxygen before they are introduced to the environmental chamber. Third, these gases must remain isolated from the ambient chamber atmosphere over the course of an experiment.
These constraints were met in the following ways in our experiments. Ten liter carboys of de-ionized water were bubbled with the ambient N2/H2 gas mixture inside the environmental chamber for several weeks prior to making stock solutions. Condensers were attached to the exit port of each carboy to minimize water loss through evaporation during this process. One molar stock solutions of FeCl2●4H2O, CaCl2●2H2O, and NaHCO3 were prepared individually from this water. Each stock solution was then bubbled with pure CO2 to replace aqueous N2/H2 with aqueous CO2 and reduce the pH sufficiently so that when aliquots of stock solution were mixed to form a master solution the solubility limit for solid carbonate was not exceeded.
One free drift experiment and two chemo-stat experiments have been conducted using the procedure described above. For the free drift experiment, a 1 L volume of master solution was mixed (see Table 2 for details) in a container having an-airtight lid with input and exits ports for gas exchange. A 3% CO2 gas mixture (N2 balance) was bubbled through the solution until gaseous-fluid equilibria was established and solution pH stabilized. Under these conditions, the master solution was nearly saturated with respect to calcite but supersaturated with respect to siderite. Aliquots of solution were immediately withdrawn for alkalinity, Fe and Ca concentration, and Fe-isotope analysis (as described in Task 2A), and 80 mg of pure calcite was introduced to the solution.
Because the solution was held at saturation with respect to calcite, minimal dissolution of the seed occurred prior to the production of a Fe-carbonate overgrowth. The overgrowth provided a protective barrier for the subsequent dissolution of the seed as the solution chemistry drifted toward equilibrium with respect to siderite over time. After 24 hours, the experiment was terminated and an aliquot of solution was filtered and analyzed for alkalinity, while two other aliquots were reserved for Fe- and Ca-concentration, and Fe-isotope analysis. The precipitated solid was separated by filtration, rinsed briefly in de-ionized water and dried in the environmental chamber for further characterization. Final solid weight was used to estimate the percentage of overgrowth. Aliquots of solid were characterized by x-ray diffraction, electron microprobe, SEM and Mössbauer spectroscopy to determine the mineralogy, Fe-content and Fe2+/Fe3+ ratio of the overgrowth. Solid will be digested for Fe and Ca analysis to confirm the mass and mole percent FeCO3 content of the overgrowth, and for Fe-isotope analysis.
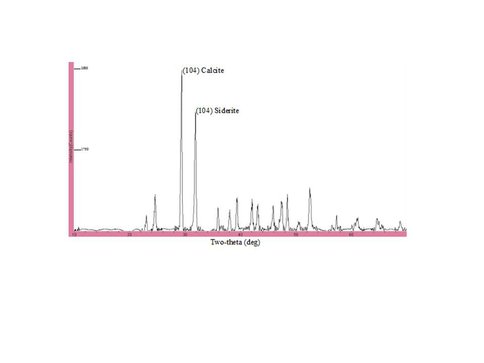
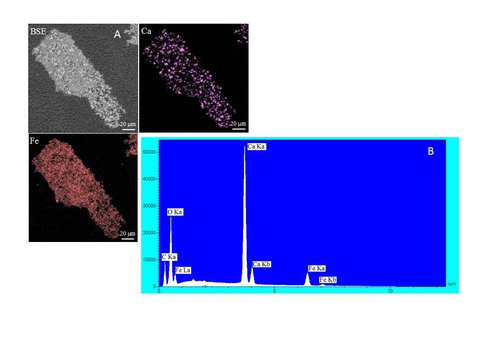
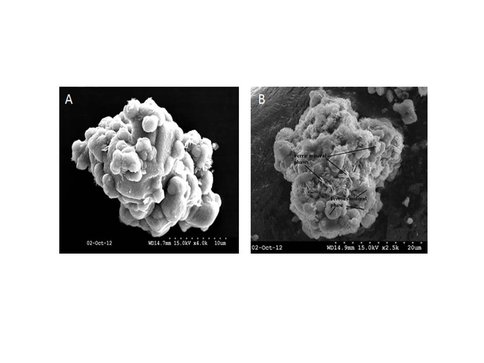
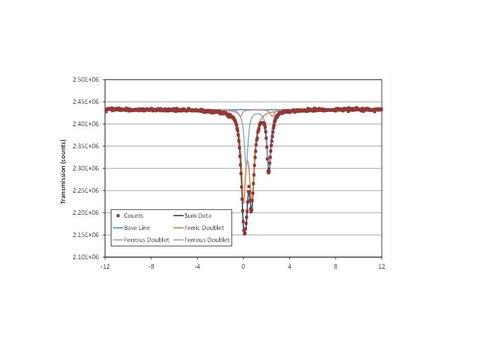
Over 140 mg of solid was generated in the free drift experiment. Siderite and calcite were the only minerals detected by XRD (Fig. 1) and electron microprobe (Fig. 2) analysis. On the other hand, SEM analysis clearly showed that a second platy phase was present on the surface of the solid (Fig. 3). Mössbauer analysis (Fig. 4) revealed two ferrous doublets that are representative of distinct bonding sites in the solid that comprise ~34% and 4% of the total Fe in the sample.
These doublets are probably representative of siderite and a second minor metastable Fe2+-bearing solid in the sample. However, Mössbauer analysis also detected a third ferric doublet that is diagnostic for a Fe3+ bonding site that contains the remaining 62% of the Fe in the sample. It is likely that the surface coating on the solid is the ferric phase detected by Mössbauer spectroscopy, although a visual estimation may suggest otherwise.
Two chemo-stat experiments were conducted under similar initial conditions (see Table 2) and they produced 185 mg and 200 mg of overgrowth. The precipitated solids were analyzed by XRD which confirmed siderite and calcite as the only two minerals in the solid, while SEM analysis (Fig. 5) showed the presence of a secondary surface coating. Mössbauer spectra are presently being collected on these solids to confirm the presence of Fe3+ in the samples.
Based on the results of the free drift and chemo-stat experiments there are two likely explanations for the existence of Fe3+ in these samples: 1) Fe oxidation of siderite occurred during the drying of samples in the environmental chamber through photo-oxidation or reaction with trace amounts of gaseous oxygen, or 2) that siderite oxidation occurred during the collection of the Mössbauer spectra. The potential also exists that Fe-oxidation involved a reactive metastable phase that was not detectable by XRD. Each of these scenarios will be tested in future experiments to resolve issues related to Fe oxidation before further experiments will be conducted. These results will form the basis for a broader suite of experiments to be completed to more fully characterize the effects of solution chemistry, precipitation kinetics, and temperature on Fe-isotope fractionation in aqueous systems that contain siderite.
Noteworthy results include:
1) Methodology for the growth of redox sensitive carbonates by the chemo-stat technique has been developed.
2) Techniques for the characterization of Fe oxidation state provide insights into potential Fe oxidation mechanisms for reactive carbonate phases.
3) Calcite seed material functions as a viable substrate for the heterogeneous growth of siderite from aqueous solution.
XRD diffractogram for solid from free drift experiment showing reflections only for calcite and siderite.
A) Backscatter electron image (BSE) and wavelength dispersive X-ray maps (Ca and Fe) of the final solid (80% overgrowth) produced during the free drift experiment. For this particular grain, the seed material forms an aggregate that bound by siderite cement. B) Energy dispersive spectroscopy (EDS) analysis of the final run product for the free drift experiment showing presence of Ca and Fe.
A. SEM image of siderite overgrowth on pure calcite seed crystal. B. SEM image showing the presence of at least two morphological phases.
Mössbauer analysis of the final solid product of the free drift experiment showing the presence of Ferrous and Ferric iron in multiple bonding environments.
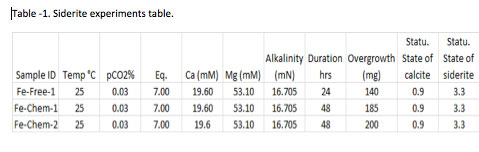
Siderite experiments table.
Publications
- Bruno, J., Wersin, P. & Stumm, W. (1992). On the influence of carbonate in mineral dissolution: II. The solubility of FeCO3 (s) at 25°C and 1 atm total pressure. Geochimica et Cosmochimica Acta, 56: 1149-1155.
- Carothers, W.W., Adami, L.H. & Rosenbauer, R.J. (1988). Experimental oxygen isotope fractionation between siderite-water and phosphoric acid liberated CO2-siderite. Geochimica et Cosmochimica Acta, 52: 2445-2450.
- Dromgoole, E.L. & Walter, M. (1990a). Iron and manganese incorporation into calcite: Effects of growth kinetics, temperature and solution chemistry. Chemical Geology, 81(4): 311-336.
- Jimenez, L.C. & Romanek, C.S. (2004). Precipitation kinetics and carbon isotope partitioning of inorganic siderite at 25oC and 1 atm. Geochimica et Cosmochimica Acta, 68: 557-571.
- Singer, P.C. & Stumm, W. (1970). The solubility of ferrous iron in carbonate-bearing waters. Journal of American Water Works Association, 62: 198-202.
- Wersin, P., Charlet, L., Karthein, R. & Stumm, W. (1989). From adsorption to precipitation: Sorption of Mn2+ on FeCO3(s). Geochimica et Cosmochimica Acta, 53: 2787-2796.
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Suvankar Chakraborty
Postdoc
Nicholas Levitt
Doctoral Student
Sagarika Banerjee
Graduate Student
Justin Abney
Undergraduate Student
-
RELATED OBJECTIVES:
Objective 4.1
Earth's early biosphere.
Objective 7.1
Biosignatures to be sought in Solar System materials