2012 Annual Science Report
 University of Wisconsin
Reporting | SEP 2011 – AUG 2012
University of Wisconsin
Reporting | SEP 2011 – AUG 2012
Project 2A: Inorganic Growth of Mg-Calcite From Dilute Solutions: Understanding Temperature, Composition and pCO2 Effects
Project Summary
The magnesium content of calcite has been successfully used in the past as a paleotemperature indicator for both inorganic and biological systems. More recently, the magnesium isotope ratio of Mg-bearing calcite has been suggested to display a temperature dependence, lending itself as an additional potential proxy for temperature. While these systems provide a valuable function to place geological materials within an environmental context, it is important to understand the physicochemical factors that control the incorporation of magnesium in calcite. Toward this end, a series of laboratory experiments were conducted to better understand the effect of solution chemistry and precipitation kinetics on the temperature dependence of the incorporation of magnesium isotopes in calcite. The results suggest that solution Mg/Ca ratio plays a subtle but important role in the use of magnesium as a paleotemperature indicator for calcite.
Project Progress
Year five accomplishments center on the inorganic growth of magnesium calcite from solution. Thirty chemo-stat experiments (Fig. 1) were performed to better understand the effect of temperature, chemical composition, pCO2, pH and precipitation rate on Mg isotope fractionation in aqueous systems that contain magnesium calcite.
One liter containers of 1.0 M stock solution of MgCl2•6H2O, CaCl2•2H2O, and NaHCO3 were prepared and bubbled with pure CO2(g) until chemical equilibrium with the gas phase was achieved (determined by constant solution pH). Individual solutions of targeted chemical composition were mixed from these stock solutions and deionized water (18 MΩ cm) to form master solutions, from which precipitation experiments were conducted. A Mg:Ca ratio of ~3.33:1 was used for 19 chemo-stat experiments and while a ratio of 10:1 was used for 11 experiments. The alkalinity of each master solution was fixed at 0.010 ± 0.002 N. Titrant solutions were prepared in an analogous fashion, with solution chemistry tailored to contribute excess ions for the precipitation reaction while maintaining a fixed chemical composition for the master solution. Prior to an experiment, the master and titrant solutions were re-equilibrated with a CO2/N2 gas mixture at a specified temperature to readjust the pH of the master solution to attain a metastable state of supersaturation with respect to Mg-calcite at a pre-selected pCO2. The physiochemical composition of the solution was adjusted so the master solution was incapable of spontaneous nucleation over the duration of an experiment but sufficiently supersaturated to support the heterogeneous growth of a solid on a pre-existing carbonate template. To initiate an experiment, calcite seed (~80 mg; Fig. 2) was added to the master solution to initiate the precipitation of Mg-calcite overgrowths. The composition of master solution was maintained by the synchronized and constant injection of two titrants (one containing aqueous Mg and Ca, and the other dissolved inorganic carbon) into the master solution by a peristaltic pump. At the conclusion of an experiment, solid was separated from the solution by filtration (0.45 µm) and an aliquot of solution was immediately analyzed for alkalinity. A second aliquot was acidified for Ca and Mg analysis by ICP-MS to confirm the chemical composition of the fluid, and a third aliquot of acidified solution was reserved for Mg isotope analysis at the University of Wisconsin. The solid run products were analyzed by x-ray diffraction (Fig. 3), scanning electron microscopy (SEM: Fig. 4), electron microprobe using WDS detector (Fig. 5) and field emission scanning electron microscopy (FESEM: Fig. 6) to visualize and quantify the mole% MgCO3 in the overgrowth. The final solid was also digested for Ca and Mg analysis by ICP-MS to estimate the mole% MgCO3 in the solid. Experimental conditions for each experiment are reported in Table 1.
Results
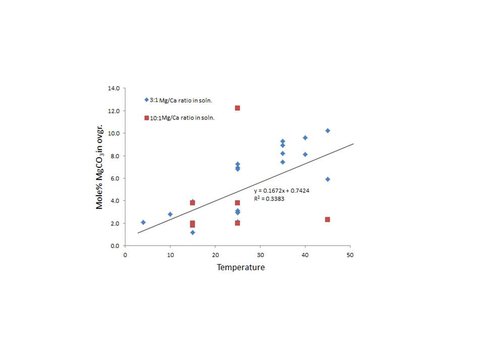
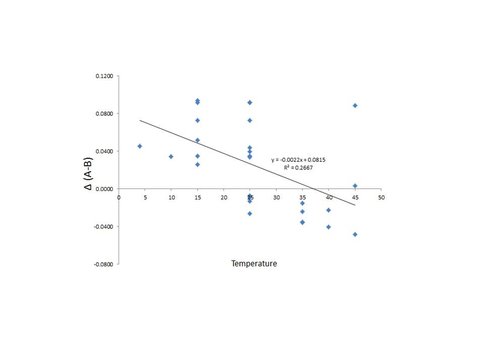
No relationship was observed between pCO2 and mole% MgCO3 in overgrowth which is consistent with the results reported by Hartley and Mucci (1996), nor was a relationship observed between precipitation rate and mole% MgCO3 in overgrowth. The results show an inverse relationship between solution Mg/Ca ratio and precipitation rate, which is consistent with results reported by Zhang and Dawe (2000). A positive relationship was observed between temperature and the mole% MgCO3 in overgrowth (Fig. 7; slope = 0.1672) which is consistent with the results reported by Lopez et al. (2009; slope = 0.1846). However, this relationship is influenced by the Mg/Ca ratio of the solution as well. According to Mucci and Morse (1983), the distribution coefficient (D) for Mg in calcite is constant above an aqueous Mg:Ca ratio of ~7.5, while below this value D increases with a decrease solution Mg:Ca ratio. Two solution Mg:Ca ratios were used in these experiments, 10:1 and 3.33:1, for which DMg values of 0.0123 and 0.0195 may be used to predict the Mg/Ca ratio in the overgrowth, respectively. The difference between the predicted and observed value for the Mg/Ca ratio in the overgrowth is compared to temperature in Fig. 8, providing a more meaningful estimate for the temperature dependence of Mg incorporation in calcite. Below 25°C, the predicted Mg/Ca ratio in the solid is higher than the observed value whereas above 25°C the predicted Mg/Ca ratio is less than the observed value.
The results will help to better constrain the temperature dependence of Mg- isotope fractionation in calcite (e.g. Li et al., 2012) once solution and solid Mg- isotope analyses are completed.
References-
Hartley, G., and Mucci, A. (1996). The influence of PCO2 on the partitioning of magnesium in calcite overgrowths precipitated from artificial seawater at 25° and 1 atm. total pressure, Geochim. Comochim. Acta., 60: 315-324.
Lopez, O., Zuddas, P., and Faivre, D. (2009). The influence of temperature and seawater composition on calcite crystal growth mechanisms and kinetics: Implications for Mg incorporation in calcite lattice, Geochim. Comochim. Acta., 73: 337-347.
Mucci, A., and Morse, J. W. (1983). The incorporation of Mg2+ and Sr2+ into calcite overgrowths: influences of growth rate and solution composition, Geochim. Comochim. Acta., 47: 217-233.
Zhang, Y., and Dawe, R. (2000). Influence of Mg2+ on the kinetics of calcite precipitation and calcite crystal morphology, Chemical Geology, 163: 129-138.
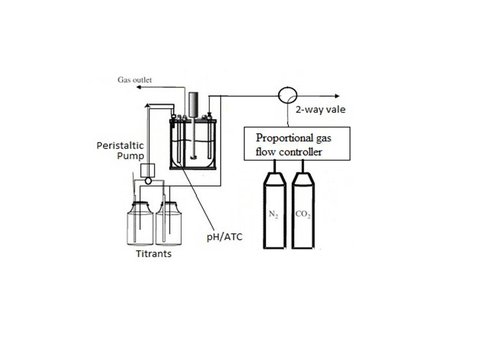
Schematic of chemo-stat system. Titrants are fed into the master solution
(shown here as water-jacketed reaction vessel) to compensate for the uptake of ions by the precipitation of carbonate, thereby maintaining a near constant pH. A CO2/N2 gas mixture is bubbled through the master solution and titrants to maintain chemical and isotope equilibrium between aqueous and gaseous phases in the system.
SEM image of pure calcite seed crystal.
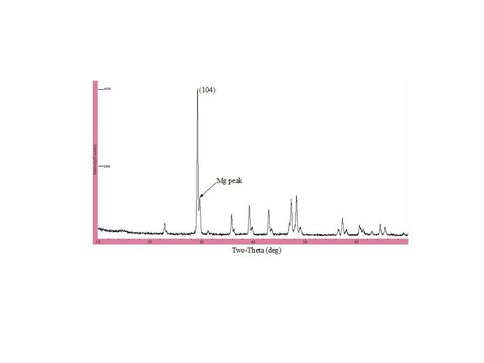
XRD diffractogram for solid from experiment Chem-26 (pH = 7.56; 35ºC; 1.65% PCO2; 20% overgrowth; 9.1 mol% MgCO3). The pattern shows a distinct shoulder on the (104) calcite peak which is diagnostic for a ~9 mol% MgCO3 overgrowth.
Mg- calcite overgrowth on pure calcite seed showing rough exterior crystal faces.
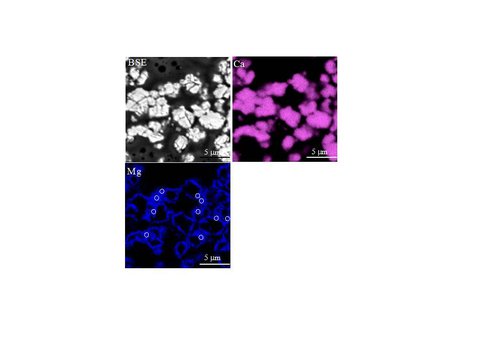
Backscatter electron image (BSE) and wavelength dispersive X-ray maps (Ca and Mg) of the final solid (20% overgrowth) produced during experiment Chem- 26 (see Fig. 4 for details of experiment). White circles are independent WDS spot analyses of the overgrowth. Twenty six WDS analyses of the overgrowth were used to quantity the mole% MgCO3 in the overgrowth for this experiment.
Secondary electron image showing spatial resolution (~5 analyses per micron) for EDS analysis of Mg-calcite overgrowth by field emission scanning electron microscopy.
Relationship between temperature and mole% MgCO3 in overgrowth.
Plot of temperature vs. difference between predicted (A) and observed (B) Mg/Ca ratio in overgrowth.
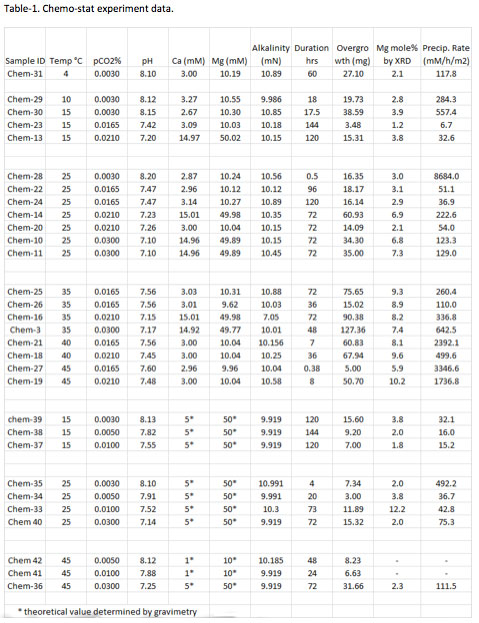
Chemo-stat experiment data.
Publications
-
Lopez, O., Zuddas, P., & Faivre, D. (2009). The influence of temperature and seawater composition on calcite crystal growth mechanisms and kinetics: Implications for Mg incorporation in calcite lattice. Geochimica et Cosmochimica Acta, 73(2), 337–347. doi:10.1016/j.gca.2008.10.022
- Hartley, G. & Mucci, A. (1996). The influence of PCO2 on the partitioning of magnesium in calcite overgrowths precipitated from artificial seawater at 25° and 1 atm total pressure. Geochimica et Cosmochimica Acta, 60: 315-324.
- Mucci, A. & Morse, J.W. (1983). The incorporation of Mg2+ and Sr2+ into calcite overgrowths: influences of growth rate and solution composition. Geochimica et Cosmochimica Acta, 47: 217-233.
- Zhang, Y. & Dawe, R. (2000). Influence of Mg2+ on the kinetics of calcite precipitation and calcite crystal morphology. Chemical Geology, 163: 129-138.
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Suvankar Chakraborty
Postdoc
Nicholas Levitt
Doctoral Student
Sagarika Banerjee
Graduate Student
Justin Abney
Undergraduate Student
-
RELATED OBJECTIVES:
Objective 4.1
Earth's early biosphere.
Objective 7.1
Biosignatures to be sought in Solar System materials