2012 Annual Science Report
 NASA Jet Propulsion Laboratory - Titan
Reporting | SEP 2011 – AUG 2012
NASA Jet Propulsion Laboratory - Titan
Reporting | SEP 2011 – AUG 2012
Task 3.3.2 Precipitation of Organics in Titan Lakes
Project Summary
Laboratory experiments simulating processes on the beaches of Titan lakes have been pursued. Work to date suggests that, at 94 K and 1 bar pressure, the precipitation of dissolved acetylene and benzene results in the formation of both solids on the beaches.
Project Progress
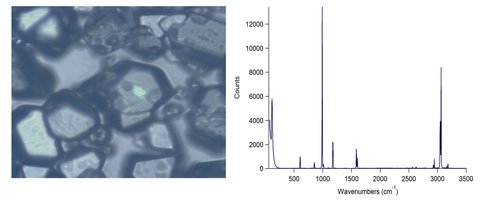
Co-Investigators Robert Hodyss and Patricia Beauchamp and Colleague Mathieu Choukroun have continued experiments designed to study the process of crystallization and precipitation in evaporating liquid ethane pools, as are likely to be found on Titan. A custom cryostat has been built for these experiments. The cryostat sample chamber is filled with ~ 5 mL of methane or ethane, and saturated with the solute or solutes of interest, then held at 94 K while a stream of nitrogen gas is blown across the surface to hasten evaporation. Experiments are performed in a nitrogen-purged glove bag in order to eliminate build-up of water ice from air. The materials remaining after evaporation are deposited onto a glass slide and analyzed by Raman spectroscopy within a Linkam LTS-350 liquid-nitrogen cryostage cooled to 94 K. The Raman spectrometer, a Horiba Jobin-Yvon LabRam HR, coupled to both a solid-state frequency doubled Nd:YAG 532 nm laser and a He-Ne 633 nm laser, has a spectral resolution of ~1 cm-1 across the spectral range of interest (100-3500 cm-1). Preliminary experiments have focused on crystallization of benzene and acetylene for characterization purposes (Figure 1).
Further experiments have been conducted by dissolving both acetylene and benzene in liquid ethane in the cryostat. Ethane is evaporated by a constant flow of gaseous nitrogen, and the precipitates formed collected on a window for micro-Raman analysis. The results of this analysis are shown on Figure 2. All spectra have been obtained at 94 K. The liquid ethane spectrum has been acquired by focusing the laser beam on top of the liquid layer. The two spectra of solid acetylene and solid benzene come from the aforementioned experiments. The bottom spectrum is obviously a mixture of the above three, without any significant change in peak position. This suggests that, at 94 K and 1 bar pressure, the precipitation of dissolved acetylene and benzene results in the formation of both solids.
Figure 1. Left: Microscope image of benzene crystals. Right: Raman spectrum of the center benzene crystal.
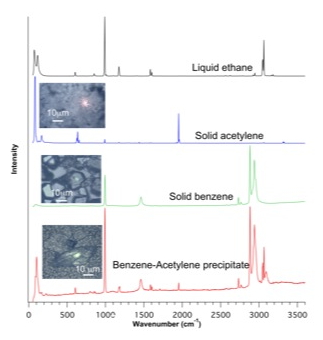
Figure 2. Images and Raman spectra of the various solid formed upon precipitation from liquid ethane, whose spectrum is shown for reference.
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Patricia Beauchamp
Co-Investigator
Mathieu Choukroun
Research Staff
-
RELATED OBJECTIVES:
Objective 2.2
Outer Solar System exploration
Objective 3.1
Sources of prebiotic materials and catalysts