2012 Annual Science Report
 NASA Jet Propulsion Laboratory - Titan
Reporting | SEP 2011 – AUG 2012
NASA Jet Propulsion Laboratory - Titan
Reporting | SEP 2011 – AUG 2012
Task 3.3.1 Solubility of Gases and Organics in Liquid Methane and Ethane
Project Summary
A goal is to measure the solubilities of Titan surface and atmospheric species in cryogenic liquid hydrocarbons, in order to constrain the composition of the hydrocarbon lakes, and provide an understanding into the nature of erosion and sedimentation on Titan.
Project Progress
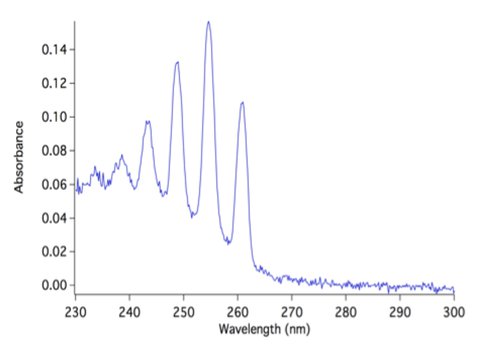
Co-Investigators Robert Hodyss, Christophe Sotin and Patricia Beauchamp and Colleague Mathieu Choukroun have continued measurements of the solubility of gases in liquid ethane and methane, using the quadrupole mass spectrometer system that was developed in the first year of this work, and investigated the implications of these results. Briefly, a quadrupole mass spectrometer (SRS 200) in a small vacuum chamber is used to sample liquids through a capillary into the mass spectrometer. Liquid drawn into the capillary will rapidly vaporize and be analyzed with the mass spectrometer. Saturated solutions of rare gas are made by first condensing ethane in a LN2 cooled, temperature-controlled dewar, then bubbling the rare gas of interest through the solution until saturation is reached. The temperature dependence of the solubilities of argon liquid ethane and methane were determined by this method. Figure 1 shows the data obtained for the temperature dependence of argon in liquid ethane. The solubilities of several small aromatics and oxygenated organics are also currently being measured, using ultraviolet absorption spectroscopy. A representative spectrum is shown in Figure 2. Both methods of sampling and analysis of liquid hydrocarbon solutions could also be easily adapted for in situ Titan missions with the goal of sampling Titan’s lakes.
In the context of this work, they have developed an ideal solution model, which uses Cassini SAR data on the extent of lakes on Titan’s surface and Huygens GCMS data on the atmospheric composition, to derive equilibrium concentration of Ar in Titan’s lakes. This model allows for estimating the total amounts of 40Ar that may be trapped in solution in the lakes. The results are compared with the total Ar budget of Titan (Figure 3).
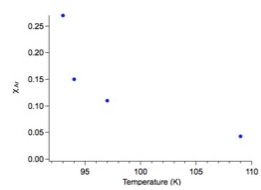
Figure. 1. Temperature dependence of the solubility of Ar in liquid ethane.
Figure 2. UV spectrum of benzene in liquid methane.
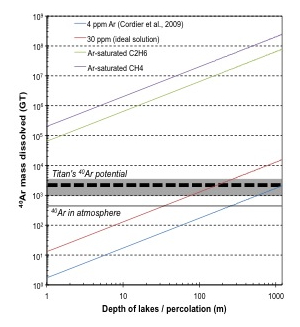
Figure 3. Mass of 40Ar dissolved in liquids as a function of depth of the lakes, which for values greater than 50-100m is taken as a proxy for the depth to which liquids percolate the icy crust beneath. The current amounts of 40Ar present in the atmosphere are indicated by the thin solid black line. The grey shaded area and the thick dashed line represent the total range of 40Ar amounts possible for Titan and the expected amount, respectively.
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Patricia Beauchamp
Co-Investigator
Christophe Sotin
Co-Investigator
Mathieu Choukroun
Research Staff
-
RELATED OBJECTIVES:
Objective 2.2
Outer Solar System exploration
Objective 3.1
Sources of prebiotic materials and catalysts