2012 Annual Science Report
 NASA Jet Propulsion Laboratory - Titan
Reporting | SEP 2011 – AUG 2012
NASA Jet Propulsion Laboratory - Titan
Reporting | SEP 2011 – AUG 2012
Executive Summary
A series of coupled model simulations and novel laboratory experiments comprise the core research program of the NAI Titan team. The objective of this coordinated research is to understand the extent to which processes that could be active currently in Titan could lead to the formation of significant prebiotic molecular compounds, to be defined hereinafter as being composed of atoms of hydrogen, carbon, nitrogen, and oxygen. These processes might have been important in the early Earth environment and be on the path to the formation of life.
The NAI Titan research program is organized along the lines of three research themes—“Titan’s geology—places where organic chemistry can operate”, “The complexity of atmospheric organic chemistry”, and “The evolved chemical state of the Titan surface”. Note that the last half of reporting period covers the first half of the fourth project year for the NAI Titan team, during which time funding for Themes 1 and 2 was sharply reduced, consistent with the selected proposal plan, in favor of enhanced funding for Theme 3.
An overview of what has occurred in each of the themes is presented in what follows.
Theme 1 – Titan’s geology—places where organic chemistry can operate:
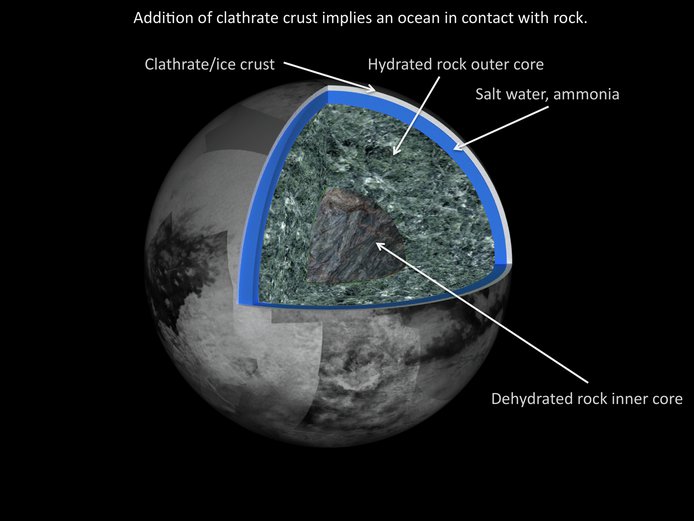
In pursuit of the theme science objectives and building on modeling done last year, the effort focused on establishing the geologically-determined conditions for organic evolution in the surface and interior. Using the standard prescription for modeling of satellite interiors, novel equations of state and thermal parameters were included that more realistically simulate Titan’s interior. The new value of the tidal love number for Titan was considered, along with inclusion of crustal clathrate. The likelihood of direct contact between an interior ocean and rock, as well as the cycling of hot water from rock to ocean, was demonstrated (Figure 1). The result raises the interest of Titan as an astrobiologically significant object in the solar system.
Studying the morphology and composition of Titan’s northern lakes and seas allow refining the values of the carbon contained in different reservoirs (atmosphere, lakes, dune fields). From this a carbon cycle can be constructed that takes into account the interactions between the interior, surface, and atmosphere (Figure 2).
In the clathrate phase, there can be an exchange between primordial methane clathrates, which are believed to make at least part of Titan’s upper crust, and ethane, which is the primary product of Titan’s atmospheric chemistry and is one of the major constituents of the lakes. Including these exchanges in a geophysical model of Titan allows explaining Titan’s shape as measured by the Cassini mission. These exchanges also may have an influence on Titan’s hydrocarbon cycle.
A Titan lake simulation system is under construction to provide a testbed for testing small instruments and components at Titan lake conditions in preparation for future in-situ missions.
Theme 2 – The complexity of atmospheric organic chemistry:
The development of a master atmospheric model is nearing completion. The condensation of molecules onto grains and sublimation back to the gas was one missing element. A new approach was developed to describe sublimation and condensation that is numerically stable and relies on the extensive database of laboratory data on the saturation vapor pressures of molecules. This, combined with the new description of grain size distribution (implemented last year) will provide a great improvement in the description of the organic chemistry in Titan’s atmosphere.
The observed vertical profiles of Titan organic aerosols have been analyzed and the possible chemistry forming these aerosols studied. The elementary reaction of the ethynyl radical with diacetylene represents an efficient pathway to produce triacetylene in Titan’s atmosphere in those regions where density profiles of photolytically-generated ethynyl radicals and diacetylene overlap. The model of Titan’s atmosphere indicates that successive reactions of the triacetylene molecule can yield even more complex polyynes. These polyynes are thought to be the basis out of which the organic aerosols are formed.
To support the master atmospheric model, realistic Titan atmospheric profiles [winds, temperatures and densities] from the surface to ~1200km, for a variety of seasons and solar cycles, is needed. The approach pursued was to combine results from a lower/middle atmosphere Titan model [TitanWRF], extending from the surface to ~430km, with results from an upper atmosphere model [such as the TGCM, run by Ingo Müller-Wodarg at ICL], which extends from ~600 to >1200km. This effort will provide (a) realistic surface to 400km or 500km datasets from two Titan general circulation models, and (b) at least one pseudo-coupled dataset extending from the surface to 1200km (Figure 3).
The search for airglow from Titan’s atmosphere during eclipse when the absence of solar XUV would allow for an accurate determination of excitation by precipitating charged particles from the magnetosphere or from cosmic rays. Titan was observed by both the Cassini ISS (Imaging Science Subsystem) at visible wavelengths and by UVIS (Ultraviolet Imaging Spectrograph) at UV wavelengths during three occasions near equinox when Titan was in Saturn’s shadow. Airglow emissions during eclipse at both UV and visible wavelengths were detected.
One project goal has been to elucidate the mechanisms and develop a quantitative understanding of particle formation and growth in the Titan atmosphere. Work has focused on elucidating the role of molecular interactions in growth of Titan aerosol particles using numerical simulations. Substantial enhancement in condensational fluxes was found for both polar and nonpolar vapor species condensing on neutral particles below 5 nm in diameter. Flux enhancements were larger in the case of charged particles, and extended over all particle sizes for polar molecule condensation.
Investigations of the condensed phase chemistry of Titan’s atmospheric aerosols has continued. The focus this year has been on the photochemistry of acetylene imbedded in C4N2 ice—to simulate atmospheric aerosol photochemistry involving most abundant unsaturated molecule (acetylene) in Titan’s atmosphere.
Theme 3 – The evolved chemical state of the Titan surface:
One focus is on understanding reactions occurring on Titan’s surface with an emphasis on determining whether mineral deposits from meteoritic infall can catalyze the formation of more complex molecules of prebiotic relevance such as amines, polyamines or simple amino acids. The project also involves understanding HCN chemistry on and within hydrocarbon ices that result from photon and electron excitations sources energy available on Titan and within Titan’s atmosphere.
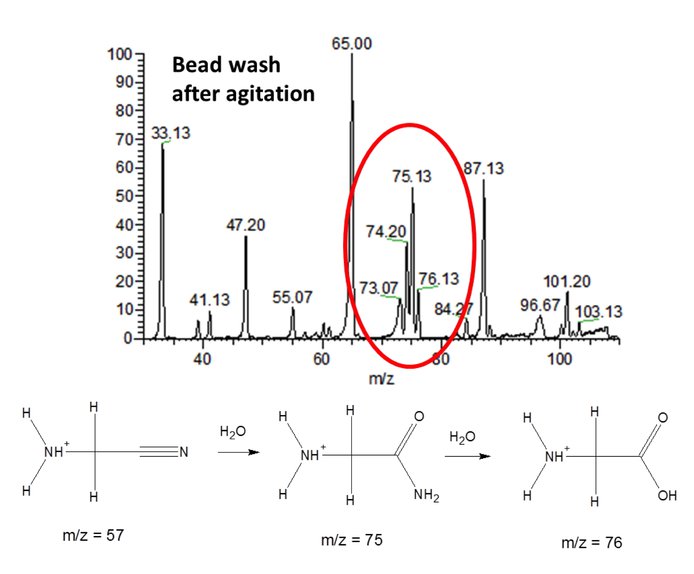
The goal of this project is to demonstrate that chemical reactions can occur between atmospheric organics and water ice, even at the low temperature (ca. 100 K) of Titan’s surface, leading to the incorporation of oxygen to form molecules of astrobiological significance. Studies using a low temperature fluidized bed reactor demonstrate that amino acids, glycine specifically, are produced by tribochemical reactions driven by Aeolian processes in Titan’s dunes (Figure 4).
Work has continued with regard to the evolution of complex organic species in astrobiologically significant regions on Titan’s surface. Oxygenation chemistry involving the condensed Titan’s organic aerosols with water-ice on Titan’s surface—induced by high energy photons simulating the cosmic ray induced chemistry on Titan’s surface—has been investigated.
Widespread lakes of liquid methane and ethane were discovered on Titan by the Cassini mission in 2006, which naturally motivates questions about the solubility of surface materials in the liquid. A goal is to measure the solubilities of Titan surface and atmospheric species in cryogenic liquid hydrocarbons, in order to constrain the composition of the hydrocarbon lakes, and provide an understanding into the nature of erosion and sedimentation on Titan. Two different experimental approaches for the determination of solubilities in liquid hydrocarbons have been pursued. The first approach is to determine solubilities via ultraviolet (UV) absorption spectroscopy of solutions of liquid methane and appropriate solutes; the second approach uses a mass spectrometer with a capillary “sipper” to analyze simulated lake fluids. To date, the solubilities of argon, krypton, and nitrogen in liquid methane and ethane, and the solubilities of toluene, benzene and naphthalene in liquid methane have been measured. Relatively high organic solubilities suggest that liquid hydrocarbon based weathering and sorting of surface organics should be occurring on Titan.
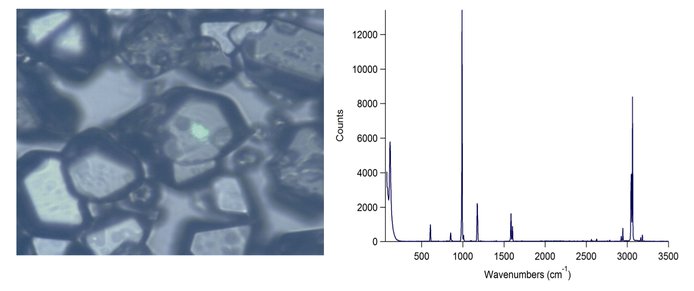
Laboratory experiments simulating processes on the beaches of Titan lakes have been pursued. A custom cryostat has been built for this investigation. Further experiments have been conducted by dissolving both acetylene and benzene in liquid ethane in the cryostat. Ethane is evaporated by a constant flow of gaseous nitrogen, and the precipitates formed collected on a window for micro-Raman analysis. The results of this analysis are shown on Figure 5. Work to date suggests that, at 94 K and 1 bar pressure, the precipitation of dissolved acetylene and benzene results in the formation of both solids on the beaches.
One effort is to develop techniques for analyzing the structural features of Titan organic aerosol analogues (tholins) and HCN polymers. This experimental program employs multinuclear and multidimensional NMR by using 13C and 15N full isotopically substituted organics, combining high revolution mass spectrometry. The most abundant small molecules and the structural features of polymeric compounds in both Titan tholins and HCN polymers have been identified.
The goal of this project has been to develop methods to identify and determine the structure of complex organic compounds formed in the Titan environment. DART (direct analysis in real time) has been demonstrated to be an effective method to employ with mass spectrometry to analyze laboratory-produced Titan tholins with a minimum of sample handling and preparation. It is anticipated that the methodology also will be useful for in situ chemical analyses in future robotic explorations of Titan’s complex atmospheric and surface chemical environment.
One project seeks to determine what chemical structures might support the genetic component of Darwinian evolution in Titan environments. The first approach is theoretical, and includes generating hypotheses for biopolymers that might dissolve in, be formed in, and remain stable in two Titan environments. The larger portion of the activities, however, has been experimental, synthesizing versions of those biopolymers and determining their performance in various models for Titan environments. The possibility of polyarsenate biopolymers as genetic materials in a hypothetical subsurface ammonia-water eutectic on Titan has been ruled out. However, the possibility of polyethers (of two types) as genetic materials within the surface hydrocarbon oceans on Titan have not been ruled out. On the contrary, to the extent that studies of solubilities of these species in liquid propane at relatively “high” temperatures (< 200 K) are adequate models for Titan’s methane oceans, the possibility remains viable. It can, however, become less so as lower and lower temperatures are examined.
Infall to the Titan system of both interplanetary and circum-Saturnian dust and ice particles can provide exogenic fluxes of several elements, such as germanium and oxygen, which may be important in facilitating potential Titan metabolisms. There are some interesting possible chemical bases for life in liquid methane, but they may depend on the supply of some relatively rare (in terms of cosmic abundance) elemental species, such as germanium.
Figure 1. – Addition of clathrate crust implies an ocean in contact with rock.
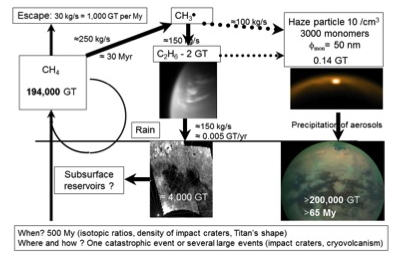
Figure 2: Summary of the carbon reservoirs that are discussed in Sotin et al. (2012). The main reservoirs are the atmospheric methane and the surface dunes. Additional reservoirs may be present in the subsurface and in the deep interior. The transformation from the original methane to the dune final product implies the formation of ethane and subsequent formation of aerosols. The amount of ethane in the atmosphere is small compared to the amount estimated in the lakes. Ethane may substitute to methane in the subsurface (Choukroun and Sotin, 2012), providing partial replenishment in atmospheric methane.
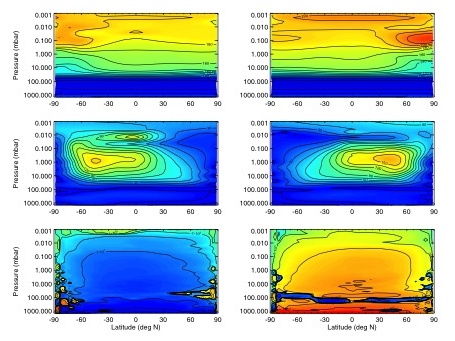
Figure 3: Titan MITgcm results showing zonal-mean temperatures (in K, top row), zonal winds (in m/s, middle row) and mass streamfunctions (in kg/s, bottom row) averaged over 12 Titan days around northern summer (Ls~90°, left column) and winter (Ls~270°, right column) solstice. Positive streamfunction values indicate clockwise rotation.
Figure 4. Mass spectrum showing organics extracted from fluidized bed reactor. Water ice reacts with aminoacetonitrile to form acetamide and glycine (observed as protonated species at m/z 75 and 76).
Figure 5. Left: Microscope image of benzene crystals. Right: Raman spectrum of the center benzene crystal.
Publications
-
Béghin, C., Randriamboarison, O., Hamelin, M., Karkoschka, E., Sotin, C., Whitten, R. C., … Simões, F. (2012). Analytic theory of Titan’s Schumann resonance: Constraints on ionospheric conductivity and buried water ocean. Icarus, 218(2), 1028–1042. doi:10.1016/j.icarus.2012.02.005
-
Choukroun, M., & Sotin, C. (2012). Is Titan’s shape caused by its meteorology and carbon cycle?. Geophysical Research Letters, 39(4), n/a–n/a. doi:10.1029/2011gl050747
-
Cornet, T., Bourgeois, O., Le Mouélic, S., Rodriguez, S., Lopez Gonzalez, T., Sotin, C., … Nicholson, P. D. (2012). Geomorphological significance of Ontario Lacus on Titan: Integrated interpretation of Cassini VIMS, ISS and RADAR data and comparison with the Etosha Pan (Namibia). Icarus, 218(2), 788–806. doi:10.1016/j.icarus.2012.01.013
-
Cornet, T., Bourgeois, O., Le Mouélic, S., Rodriguez, S., Sotin, C., Barnes, J. W., … Nicholson, P. D. (2012). Edge detection applied to Cassini images reveals no measurable displacement of Ontario Lacus’ margin between 2005 and 2010. Journal of Geophysical Research: Planets, 117(E7), n/a–n/a. doi:10.1029/2012je004073
-
Foster, P. L. (2011). Comment on “A Bacterium That Can Grow by Using Arsenic Instead of Phosphorus”. Science, 332(6034), i–1149. doi:10.1126/science.1201551
-
He, C., Lin, G., Upton, K. T., Imanaka, H., & Smith, M. A. (2012). Structural Investigation of HCN Polymer Isotopomers by Solution-State Multidimensional NMR. The Journal of Physical Chemistry A, 116(19), 4751–4759. doi:10.1021/jp301604f
-
He, C., Lin, G., Upton, K. T., Imanaka, H., & Smith, M. A. (2012). Structural Investigation of Titan Tholins by Solution-State 1 H, 13 C, and 15 N NMR: One-Dimensional and Decoupling Experiments. The Journal of Physical Chemistry A, 116(19), 4760–4767. doi:10.1021/jp3016062
-
Lòpez-Yglesias, X., & Flagan, R. C. (2013). Population Balances of Micron-Sized Aerosols in a Bipolar Ion Environment. Aerosol Science and Technology, 47(6), 681–687. doi:10.1080/02786826.2013.783683
-
López-Yglesias, X., & Flagan, R. C. (2013). Ion–Aerosol Flux Coefficients and the Steady-State Charge Distribution of Aerosols in a Bipolar Ion Environment. Aerosol Science and Technology, 47(6), 688–704. doi:10.1080/02786826.2013.783684
-
Sotin, C., Lawrence, K. J., Reinhardt, B., Barnes, J. W., Brown, R. H., Hayes, A. G., … Stephan, K. (2012). Observations of Titan’s Northern lakes at 5μm: Implications for the organic cycle and geology. Icarus, 221(2), 768–786. doi:10.1016/j.icarus.2012.08.017
-
West, R. A., Ajello, J. M., Stevens, M. H., Strobel, D. F., Gladstone, G. R., Evans, J. S., & Bradley, E. T. (2012). Titan airglow during eclipse. Geophysical Research Letters, 39(18), n/a–n/a. doi:10.1029/2012gl053230