2012 Annual Science Report
 NASA Jet Propulsion Laboratory - Icy Worlds
Reporting | SEP 2011 – AUG 2012
NASA Jet Propulsion Laboratory - Icy Worlds
Reporting | SEP 2011 – AUG 2012
Habitability of Icy Worlds
Project Summary
Habitability of Icy Worlds investigates the habitability of liquid water environments in icy worlds, with a focus on what processes may give rise to life, what processes may sustain life, and what processes may deliver that life to the surface. Habitability of Icy Worlds investigation has three major objectives. Objective 1, Seafloor Processes, explores conditions that might be conducive to originating and supporting life in icy world interiors. Objective 2, Ocean Processes, investigates the formation of prebiotic cell membranes under simulated deep-ocean conditions, and Objective 3, Ice Shell Processes, investigates astrobiological aspects of ice shell evolution.
Project Progress
Investigation 1- Habitability of Icy Worlds Progress
Theme 1: Habitability
Objective 1: Seafloor Processes
This objective investigates conditions that might be conducive to originating and supporting life in icy world interiors. To enable prediction testing by this and other teams pursuing related questions, we will define biomarker production and look for ground-truth in analog terrestrial systems.
Project 1: Hydrothermal reactor experiments for origin of life Investigators (Co-Is): Michael Russell (lead), Isik Kanik, Steve Vance, Gordon Love and Max Coleman.
Members: Laurie Barge, John Baross, Lance Christensen, Richard Kidd, Randall Mielke, and Lauren Spencer-White.
Research: Tests the hypothesis that life can originate through a seafloor process that occurs at temperatures lower than those occurring in hydrothermal systems at mid-ocean ridges.
Year 4 Objectives: Analyze reactor effluents and investigate Fe, S, and C biogeochemical cycles. Consider relevance of a series of geochemical and electrochemical reactions at submarine alkaline vents as plausible harbingers of metabolism and life in the oceans of Icy Worlds.
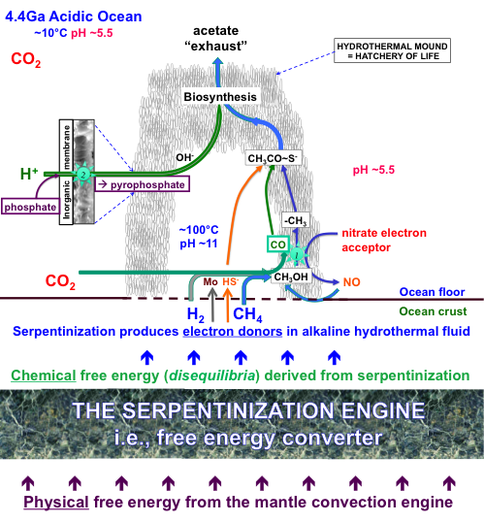
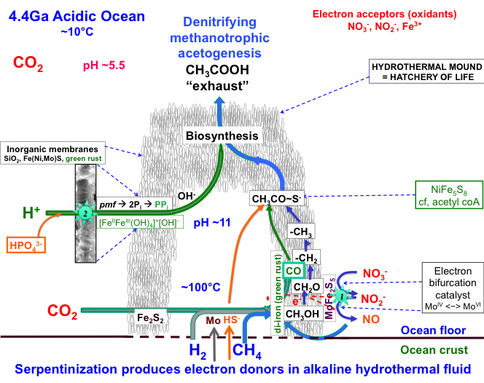
Progress: We continued to produce methane from CO2 (an atmospheric gas to be expected at high partial pressure early in the histories of wet rocky bodies (Shibuya et al., 2012, in press)) in our serpentinizing experiments involving komatiite and pyrrhotite/pentlandite (hydrothermal reactor) However, we failed to produce the thermodynamically challenging formaldehyde (HCHO). This negative result forced us to formulate an alternative pathway to this vital intermediate involving the oxidation of our hydrothermal methane with nitrate (Figure 1-1). The same waste product or effluent is to be expected from this alternative putative pathway to acetate (CH3COOH, a life detection molecule) – a process we name as “denitrifying, methanotrophic acetogenesis” (Russell et al. in press; Nitschke & Russell, in press) (Figure 1-2).
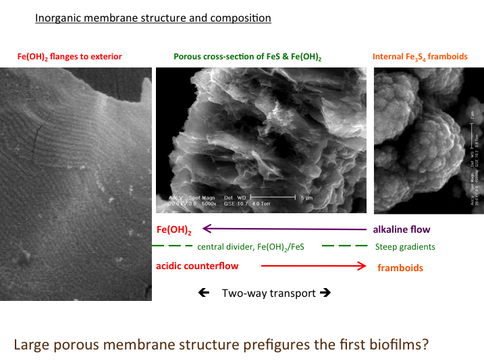
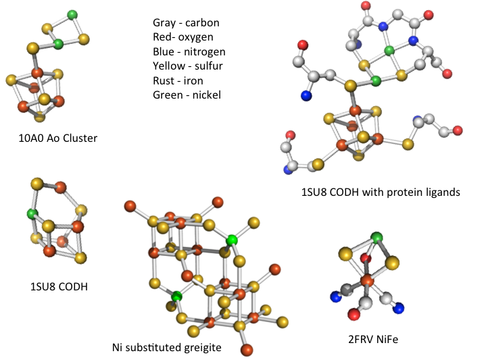
Our membrane experiments continued with the demonstration of self-organizing dissipative structures to be expected in the far-from-equilibrium conditions obtaining at submarine alkaline hydrothermal springs (Figure 1-3) (Mielke et al., 2011; Barge et al., 2012; McGlynn et al., 2012). Raman analyses of these hydrothermal chimneys using a newly fabricated rig Figure 1-4) confirmed that mackinawite (FeS) dominated the chimney walls at all temperatures, though crystal size reduced to nanocrystalline scales at 60°C and above (Figure 1-5) (White et al., 2012). Another effect of increasing temperature was the partial transformation of mackinawite to greigite (Fe3S4) which reached a maximum of 8% of the sulfide at 70°C (Figure 1-5). Within this higher range of temperatures, a further set of peaks were identified between 75°C and 80°C which matched closely to lepidocrocite (ɣ-FeOOH). We speculate that the lepidocrocite was the end product of oxidation of iron that was achieved via green rust (~[FeIIFeIII(OH)4]+[OH]-) and we are planning experiments to investigate this supposition. Were this to be the case it would add a further mineral affine with the active centers of the main metalloenzymes known on phylogenetic grounds to have been involved in biosynthetic reactions in the last universal common ancestor (LUCA). These comprise methane monoxygenase (cf. green rust or ~[FeIIFeIII(OH)4]+[OH]-), carbon monoxide dehydrogenase and acetyl coenzyme-A synthase (greigite or ~SNiS[Fe4S4]SFeS) and hydrogenase (cf. mackinawite or FeS). The sulfide structures are compared in Figure 1-6.
How serpentinization operates as the intermediary engine between a mantle convection engine or plume formation and the first metabolic engines 1 and 2 (Russell et al.2012, in press).
How denitrifying, methanotrophic acetogenesis drives the acetyl coenzyme-A pathway form either end to transcend otherwise unassailable thermodynamic barriers to carbon fixation by involving a molybdenum complex (MoIV/VI2Fe3S0/2-9) in electron bifurcation. Engine 1 is the “Carbon fixation engine” and Engine 2 is the putative “Pyrophosphatase engine” (Russell et al. 2012, in press).
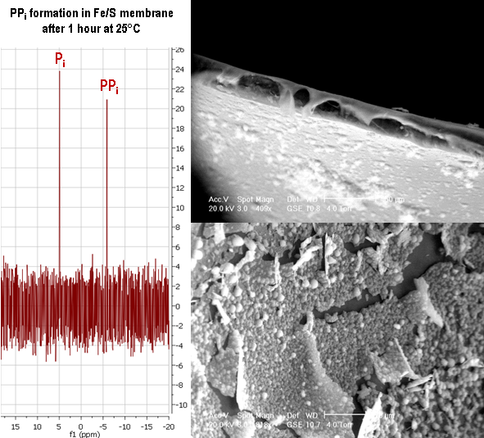
Knowing the nature of these chimneys, our next objective was to demonstrate the generation of pyrophosphate from monophosphate through the action of the proton gradient across these inorganic membranes as analyzed by nuclear magnetic resonance (NMR). This was achieved (Figure 1-7 and cf Figure 1-2) (Barge et al., 2012, in review).
Conclusions
Life emerges to resolve the chemical tensions on wet (icy) rocky worlds between CO2 + NO3- + Fe3+ versus H2 + CH4 and dissipate electrochemical and thermal gradients. In particular it hydrogenates CO2 and to a subordinate degree, oxidizes abiotic methane to produce a small but continuous supply of organic molecules and increases entropy overall. It begins the process through the spontaneous production of a materials and a catalytic free energy (entropy) trap on the ancient ocean floor at an alkaline spring derived by serpentinization.
Life should be considered as the evolutionary culmination of emergent symmetry-breaking, macroscopically organized dynamic structures in the Universe. Members of this cascading series of autocatalytic energy converting systems [i.e., engines] become ever more complex-more chemical and less physical-as each engine extracts, exploits and generates ever lower grades of energy and resources in the service of entropy generation. Each one of these engines emerges spontaneously from order created by a particular mother engine or engines.
A composite selection of micrographs illustrating the regular flanges that comprise ferrous hydroxide micro-dendrites on the exterior of an iron sulfide–bearing membrane (left), a cross section (middle), and framboidal iron sulfides from the interior of a chimney (right). We speculate that flange growth results from the escape of alkaline solution through the membrane along subcompartments oriented normal to growth. Where this solution meets the ferrous iron, ferrous hydroxide is precipitated as ribs, also normal to growth. A counterflow of protons down-gradient to the alkaline interior takes place in the neighboring subcompartments. Platelets of ferrous minerals are spontaneously precipitated at the interface of the two solutions. This is where we imagine the potential exists for carbon dioxide reduction/hydrogenation and pyrophosphate condensation under the higher pressures obtaining early in the history of wet, rocky worlds.
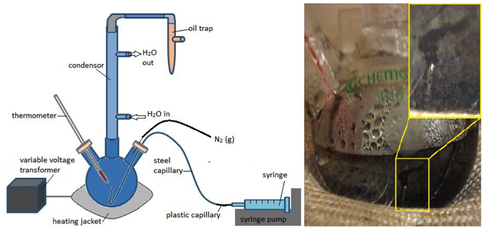
Diagram of apparatus used for heated chimney precipitation experiments (left). A digital image (right) reveals a single inorganic chimney precipitated from the slow seepage from the syringe of alkaline fluid into the simulated acidic ocean in the round-bottom flask. (White et al., 2012, in review).
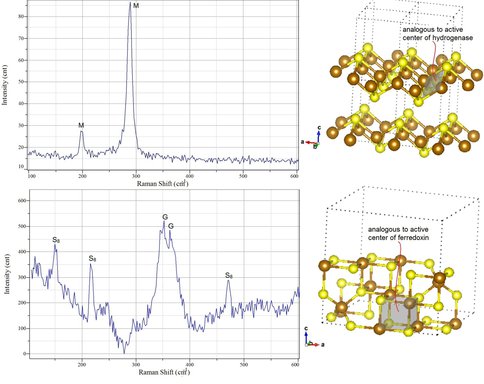
Raman spectra collected from precipitated iron sulfide chimneys reveals distinct spectra for mackinawite (M) (top left) and greigite (G) (bottom left). The mackinawite crystal structure (top right) contains rhombs which are affine to active metal centers in certain hydrogenase enzymes (cf. 2FRV NiFe hydrogenase in Figure 1-6). The greigite crystal structure (bottom right) contains a cubane structure which is similar to the active metal centers in several enzymes involved in anaerobic metabolic pathways likely first beaten at the origin of life (Figure 1-6) (Russell et al. in press and in review). Greigite [FeIIFeIII2S4] is observed in chimneys precipitated at ~70°C (White et al., 2012, in review).
The structure of greigite compared to the active metal centers in Ni-Fe hydrogenase, CO dehydrogenase (CODH) and acetyl coenzyme-A (the Ao cluster) (Russell et al. 2012, in press and Russell et al., in review).
Pyrophosphate (PPi) formation in FeS membranes (ESEM images, right) in a pH gradient. At 25°C, reaction between acidic iron/phosphate and alkaline sulfide/acetyl phosphate solutions produced FeS films containing orthophosphate. Left: After 1 hour the membrane contained ~14% PPi, relative to all precipitated phosphates as detected by 31P NMR (Barge et al. 2012, in prep.).
As a part of the seafloor project, Co-I Vance, working with Lance Christensen, conducted a field trip and sampled serpentinizing fluids and gases from The Cedars, near Cazadero, CA, and Cabeço de Vide in central Portugal. Sampled gases show signatures of ethane and methane when analyzed using the Carbon Isotope Laser Spectrometer (CILS). These gases are known to exsolved from fluids at The Cedars, but have not yet been documented at the latter site. Detection using CILS is useful as an analogue to pending investigations by TLS on SAM on the Curiosity rover. The intention for this work is to develop CILS as an instrument that can be used for rapidly characterizing and understanding serpentinizing systems and similar sites of interest as analogues to the origin of life on Earth, and on Mars and Europa. This instrumentation would also be of interest for deploying to Titan or the large outer planets in order to understand their composition and volatile processes.
Dr. Vance shared his preliminary results with Portugese media, highlighting the importance in the context of understanding life’s possible origin in serpentinizing systems on Earth, Mars, and Europa.
Objective 2: Ocean Processes
The Ocean Processes objective examines deep-ocean conditions, computationally assesses the distribution of material and heat through the ocean and ice shell, and seeks to improve relevant chemical literature through a set of high-pressure equation-of-state experiments.
Project 1: Mass and Heat Transport in icy world oceans (Co-Is): Steve Vance (lead), J. Michael Brown, Jason Goodman, Isik Kanik
Members: Jeff Collins, Erik Lenferenk
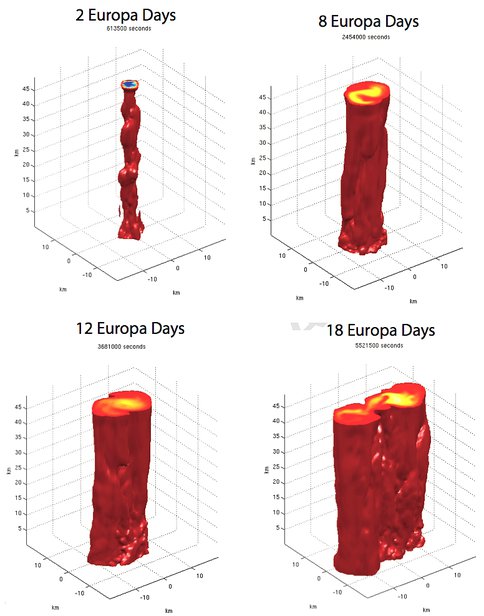
The liquid water interiors of Europa and other icy moons of the outer solar system are likely to be driven by geothermal heating from the sea floor, leading to the development of buoyant hydrothermal plumes. These plumes potentially control icy surface geomorphology, and are of interest to astrobiologists. As a part of Ocean processes project, our Co-I Jason Goodman and colleagues have performed a series of simulations of these plumes using the MIT GCM ocean circulation model. We assume here that Europa’s ocean is deep (of order 100 km) and unstratified, and that plume buoyancy is controlled by temperature, not composition. Their experiments explore a limited region of parameter space, with ocean depth H ranging from 50 to 100 km deep, source heat flux Q between 0.1 and 10 GW, and Coriolis parameter f corresponding to Europa latitudes between 9_ and 47_. As predicted by their earlier work, the plumes in their simulations form narrow cylindrical chimneys (a few km across) under the influence of the Coriolis effect. These plumes broaden over time until they become baroclinically unstable, breaking up into cone-shaped eddies when they become 10-35 km in diameter; the shed eddies are of a similar size. Large-scale currents in the region of the plume range between 1 and 5 cm/s; temperature anomalies in the plume far from the seafloor are tiny, varying between 10 and 180 microkelvin. Variations in plume size, shape, speed, and temperature are in excellent agreement with previous laboratory tank experiments, and in rough agreement with theoretical predictions. Plume dynamics and geometry are controlled by a “natural Rossby number” which depends strongly on depth H and Coriolis parameter f, but only weakly on source heat flux Q. However, some specific theoretical predictions are not borne out by the simulations: this may occur because the plumes are “reingesting” their own emissions, a process not considered in their earlier theory.
Evolution of a typical hydrothermal plume in our simulations. The red surface marks the outer boundary of the plume, where the water temperature is 10−6 K above ambient. Initially, Coriolis forces constrain the warm buoyant plume fluid to a narrow cylindrical structure. Once this structure reaches the ice/water interface, it begins to spread out laterally, forming a broader cylinder or cone. When the structure broadens beyond the critical Rossby radius of deformation, parts of it begin to break off, forming baroclinic eddies — this process has just begun in the third panel, and is well underway in the fourth. In this simulation, H = 50 km, Q = 1 GW, f = 1.3×10−5 s−1.
The recent experimental work by Goodman et al., 2012 broadly confirms the theoretical description of hydrothermal plumes given by Goodman et al. (2004). They found that plume shapes, sizes, temperatures, velocities, etc. are roughly in accord. However, plume velocities are larger, and velocities and temperatures are more sensitive to the intensity of the hydrothermal source than predicted by theory. Goodman et al., 2012 have ruled out a variety of hypotheses to explain the model/theory mismatch, but have no satisfactory explanation as yet. Figuring this out is a primary focus for their future research. They also intend to continue investigation into the nonlinear equation of state of icy world ocean brines, the effect of horizontal components of planetary rotation, the impact of hydrothermal 620 plumes on the global ocean circulation, and the detectability of these plumes using magnetometers.
Year 4 Objectives: Evaluate role of turbulence in plume dynamics. Evaluate role of precipitation in plume dynamics. Evaluate pressure and composition dependent melting effects on ice ceiling.
Progress: Prof. Goodman and his student published results from the initial study of the 3d evolution of a plume in Europa’s ocean. A model run incorporating equations of state for MgSO4 has been performed and is being prepared for publication.
Project 2: Ice world ocean chemistry (Co-Is): Steve Vance (lead), J. Michael Brown, Isik Kanik
Year 4 Objectives: Obtain equations of state (thermodynamic properties) for aqueous ammonia under deep ocean pressure, analyze and publish results, interface with computational tasks.
Progress: Vance and Brown implemented a spline-based method for generating self-consistent equations of state data for aqueous solutions. Application to existing sound velocity data from Vance’s thesis work (2007) has led to accurate representations of density, sound velocity, heat capacity, and related quantities over the full range of pressures, temperatures, and compositions relevant to icy world oceans. Initial considerations for Ganymede show that highly saline fluids are gravitationally stable under high-pressure ice layer at the base of Ganymede’s ocean, opening the possibility for water-rock interactions in large icy worlds. This work led to a joint, collaborative work with Drs. Sotin and Choukroun as part of objective 3. A publication submitted for the MGSO4 results is currently under revision.
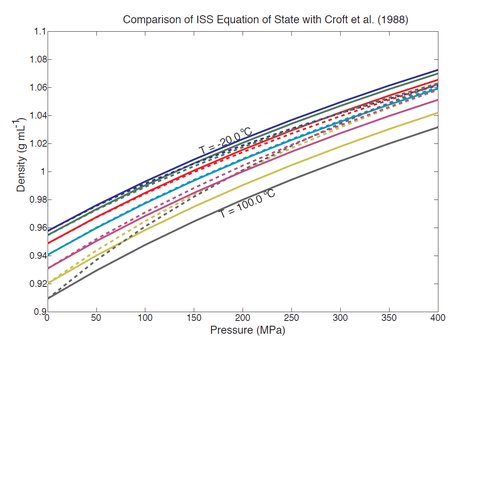
The methods and analyses described therein are being applied to sound velocity data for ammonia acquired in 2011, and a paper is being drafted for publication in 2013. Preliminary analysis shows substantially less temperature dependence in compressibility from previous predictions by Croft et al. (1988) in the context of an ocean under Titan’s surface. Results from this work will be applied to understanding Titan’s internal structure in a similar manner to the summer work for MgSO4 described in the next section. It is anticipated that ammonia should have an even stronger influence on the presence of high-pressure ice layers, and our initial hypothesis is that the effect could permit a seafloor in direct contact with ocean fluids.
Measured densities of ammonia-water mixtures (12.4 Wt%) are generally less than those for pure water, by as much as 10% the highest pressures. Results from our laboratory (solid lines) differ significantly from predictions by Croft et al. (1988; dashed lines). From Vance and Brown, 2012 submitted.
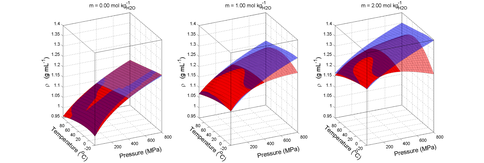
Densities for aqueous MgSO4 agree with predictions for pure water over the range of temperatures and pressures relevant to icy world oceans (-20 to 100 oC and 0.1 to 800 MPa), but show stark divergences at elevated pressures when MgSO4 is added. Concentrations shown correspond to 10 and 20 Wt%. (from Vance and Brown, 2012, submitted).
Comparison of densities for various concentrations of aqueous MgSO4 shows that the high compressibility of fluids relative to ices leads to regions in pressure and temperature in which concentrated fluids are denser than high-pressure ice, with implications for the development of transient liquid layers at the seafloor and possibly in between different phases of high pressure ice. The scenario is being referred to as the “Dagwood sandwich” ocean (from Vance and Brown, 2012, in review)
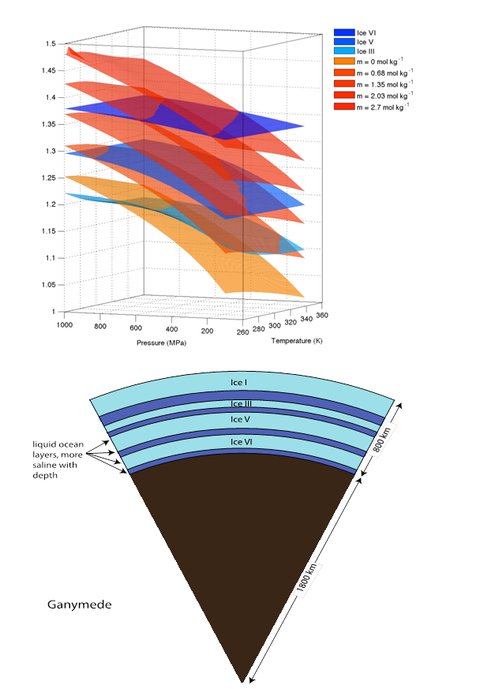
TOP: Densities of concentrated MgSO4 solutions compared with those of high pressure ice phases III, V, and VI in the range of pressure and temperatures explored in this work. Ice III is less dense than ice V, which is less dense than ice VI. All three solid phases are less compressible than the luids, which increase in density primarily with increasing pressure and concentration. BOTTOM: A “Dagwood sandwich” ocean postulated to exist in very large icy bodies with sufficient depth to create high-pressure ice layers, and with enough internal heating to create liquid water at the ice-rock interface. The above example for Ganymede uses mean H2O layer thickness for from Anderson et al. (1996) and relative ice layer thicknesses from Barr et al. (2001). Callisto and Titan, which are also thought to contain liquid water, are similar in size and could also conceivably host multiple ocean layers.
Objective 3: Ice Shell Processes
The Ocean Processes objective examines deep-ocean conditions, computationally assesses the distribution of material and heat through the ocean and ice shell, and seeks to improve relevant chemical literature through a set of high-pressure equation-of-state experiments.
Project 1: Develop multi-phase ice-shell model to assess liquids’s role in ice shell habitability / transport. Analyze and publish results, interface with computational / experimental task. Co-I’s: Mathieu Choukroun, Isik Kanik, Christophe Sotin, Steve Vance
Members: Mathieu Bouffard (Ecole Normale Superior, Lyon, France)
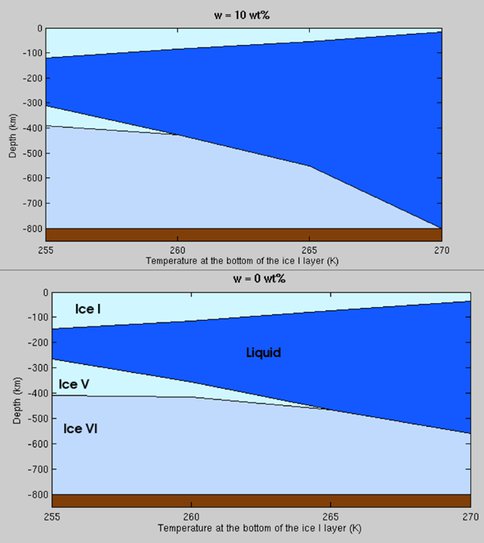
Summer student Mathieu Bouffard worked on a new thermodynamic model for the H2O – MgSO4 system under conditions relevant to the interiors of large icy satellites. He built on equations of state for MgSO4 and water ice developed by Drs. Vance and Choukroun, respectively, to develop equations of state for phase equilibria in MgSO4 oceans. Results were applied in the context of a simple model for the interior structure of Ganymede’s H2O layer, with help from Dr. Sotin. The model allows prediction of thickness of an ocean of a given composition or, conversely, prediction of ocean composition based on inferred density and thickness (as might be revealed, for example, from orbital radio science measurements by the planned JUICE mission). A theoretical result of the work is that oceans with salinity greater than 10 Wt% (about 1 molal concentration) would preclude the existence of high pressure ice layers, creating an ocean similar to Europa’s, albeit at very high pressures. Exploring the physical plausibility of such scenarios is one of the goals of work planned for the coming year and beyond.
Thicknesses of H2O layers in a model Ganymede containing an ocean with MgSO4-dominated composition. The horizontal axis tracks layer thicknesses as a function of the melting temperature of the upper ice I layer (a convenient model boundary condition). The presence of 10 Wt% MgSO4 eliminates 200 km of high-pressure ice. Starting composition would need to close to the eutectic concentration to accommodate dilution of the ocean by melted high-pressure ice. Conversely, if Ganymede’s present ocean is extensive and close to the eutectic concentration, prior epochs during which higher radiogenic heating would have been present could have seen an ocean in direct contact with its seafloor.
Publications
-
Barge, L. M., Doloboff, I. J., Russell, M. J., VanderVelde, D., White, L. M., Stucky, G. D., … Kanik, I. (2014). Pyrophosphate synthesis in iron mineral films and membranes simulating prebiotic submarine hydrothermal precipitates. Geochimica et Cosmochimica Acta, 128, 1–12. doi:10.1016/j.gca.2013.12.006
-
Barge, L. M., Doloboff, I. J., White, L. M., Stucky, G. D., Russell, M. J., & Kanik, I. (2012). Characterization of Iron–Phosphate–Silicate Chemical Garden Structures. Langmuir, 28(8), 3714–3721. doi:10.1021/la203727g
-
Branscomb, E., & Russell, M. J. (2013). Turnstiles and bifurcators: The disequilibrium converting engines that put metabolism on the road. Biochimica et Biophysica Acta (BBA) – Bioenergetics, 1827(2), 62–78. doi:10.1016/j.bbabio.2012.10.003
-
Goodman, J. C., & Lenferink, E. (2012). Numerical simulations of marine hydrothermal plumes for Europa and other icy worlds. Icarus, 221(2), 970–983. doi:10.1016/j.icarus.2012.08.027
-
McGlynn, S. E., Kanik, I., & Russell, M. J. (2012). Peptide and RNA contributions to iron-sulphur chemical gardens as life’s first inorganic compartments, catalysts, capacitors and condensers. Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences, 370(1969), 3007–3022. doi:10.1098/rsta.2011.0211
-
Mielke, R. E., Robinson, K. J., White, L. M., McGlynn, S. E., McEachern, K., Bhartia, R., … Russell, M. J. (2011). Iron-Sulfide-Bearing Chimneys as Potential Catalytic Energy Traps at Life’s Emergence. Astrobiology, 11(10), 933–950. doi:10.1089/ast.2011.0667
-
Nitschke, W., & Russell, M. J. (2011). Redox bifurcations: Mechanisms and importance to life now, and at its origin. BioEssays, 34(2), 106–109. doi:10.1002/bies.201100134
-
Nitschke, W., & Russell, M. J. (2013). Beating the acetyl coenzyme A-pathway to the origin of life. Philosophical Transactions of the Royal Society B: Biological Sciences, 368(1622), 20120258–20120258. doi:10.1098/rstb.2012.0258
-
Nitschke, W., McGlynn, S. E., Milner-White, E. J., & Russell, M. J. (2013). On the antiquity of metalloenzymes and their substrates in bioenergetics. Biochimica et Biophysica Acta (BBA) – Bioenergetics, 1827(8-9), 871–881. doi:10.1016/j.bbabio.2013.02.008
-
Russell, M. J., Nitschke, W., & Branscomb, E. (2013). The inevitable journey to being. Philosophical Transactions of the Royal Society B: Biological Sciences, 368(1622), 20120254–20120254. doi:10.1098/rstb.2012.0254
-
Schoepp-Cothenet, B., Van Lis, R., Atteia, A., Baymann, F., Capowiez, L., Ducluzeau, A-L., … Nitschke, W. (2013). On the universal core of bioenergetics. Biochimica et Biophysica Acta (BBA) – Bioenergetics, 1827(2), 79–93. doi:10.1016/j.bbabio.2012.09.005
-
Schoepp-Cothenet, B., Van Lis, R., Philippot, P., Magalon, A., Russell, M. J., & Nitschke, W. (2012). The ineluctable requirement for the trans-iron elements molybdenum and/or tungsten in the origin of life. Scientific Reports, 2. doi:10.1038/srep00263
-
Shibuya, T., Tahata, M., Ueno, Y., Komiya, T., Takai, K., Yoshida, N., … Russell, M. J. (2013). Decrease of seawater CO2 concentration in the Late Archean: An implication from 2.6Ga seafloor hydrothermal alteration. Precambrian Research, 236, 59–64. doi:10.1016/j.precamres.2013.07.010
-
Simoncini, E., Russell, M. J., & Kleidon, A. (2011). Modeling Free Energy Availability from Hadean Hydrothermal Systems to the First Metabolism. Orig Life Evol Biosph, 41(6), 529–532. doi:10.1007/s11084-011-9251-4
-
Vance, S., & Michael Brown, J. (2013). Thermodynamic properties of aqueous MgSO4 to 800MPa at temperatures from −20 to 100°C and concentrations to 2.5molkg−1 from sound speeds, with applications to icy world oceans. Geochimica et Cosmochimica Acta, 110, 176–189. doi:10.1016/j.gca.2013.01.040
- White, L.M., Bhartia, R., Stucky, G.D., Kanik, I. & Russell, M.J. (In Review). Characterizing compositional variations in catalytic iron-sulfide species in ancient alkaline hydrothermal vent systems. Earth and Planetary Science Letters.
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
John Baross
Collaborator
Mathieu Choukroun
Collaborator
Lance Christensen
Collaborator
Richard Kidd
Collaborator
Laura Barge
Postdoc
Randall Mielke
Research Staff
Lauren Spencer-White
Doctoral Student
-
RELATED OBJECTIVES:
Objective 1.1
Formation and evolution of habitable planets.
Objective 2.1
Mars exploration.
Objective 2.2
Outer Solar System exploration
Objective 3.1
Sources of prebiotic materials and catalysts
Objective 3.2
Origins and evolution of functional biomolecules
Objective 3.3
Origins of energy transduction
Objective 3.4
Origins of cellularity and protobiological systems
Objective 4.1
Earth's early biosphere.
Objective 5.1
Environment-dependent, molecular evolution in microorganisms
Objective 6.1
Effects of environmental changes on microbial ecosystems
Objective 6.2
Adaptation and evolution of life beyond Earth
Objective 7.1
Biosignatures to be sought in Solar System materials
Objective 7.2
Biosignatures to be sought in nearby planetary systems