2012 Annual Science Report
 NASA Goddard Space Flight Center
Reporting | SEP 2011 – AUG 2012
NASA Goddard Space Flight Center
Reporting | SEP 2011 – AUG 2012
Composition of Parent Volatiles in Comets: Oxidized Carbon
Project Summary
GCA Co-Investigator Dr. Michael DiSanti continued his work on measuring parent volatiles in comets using high-resolution near-infrared spectroscopy at world class observatories in Hawai’i and Chile. The goal of this work is to build a taxonomy of comets based on ice compositions, which show considerable variation among comets measured to date. For the past several years, Co-I DiSanti’s research has emphasized the chemistry of volatile oxidized carbon, in particular the efficiency of converting CO to H2CO and CH3OH on the surfaces of icy interstellar grains through H-atom addition reactions prior to their incorporation into comets. More recently, we have extended our thinking by suggesting oxidation reactions on grains as a means of interpreting results from our recent observational campaign on long-period comet C/2009 P1 (Garradd), in the fall/winter 2011/2012. We have also made major strides in the development and application of fluorescence models for interpretation of observed line intensities in comets, including an empirical treatment of the n2 band of CH3OH, led by Co-I DiSanti.
Project Progress
Overview
Our studies of native ices in comets (i.e., those contained in the nucleus) have important implications for Astrobiology. These volatiles, which we measure routinely in comets, include several important pre-biotic molecules that are thought to play significant roles in the origin of life on Earth. For example, HCN and NH3 lead to amino acids, H2CO leads to sugars, and CH4 and C2H6 are suggested as respective precursors to the methylamine and ethylamine that were unambiguously identified in Stardust samples returned to Earth from comet 81P/Wild 2.
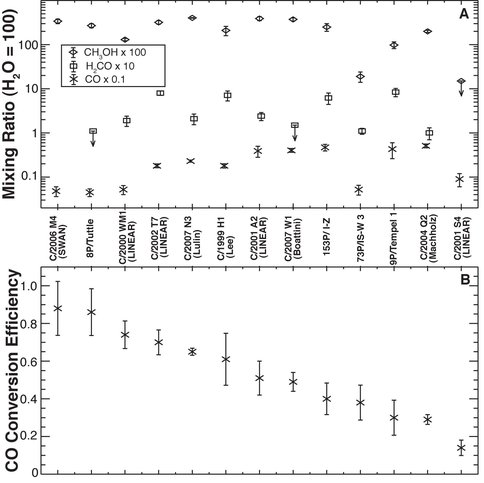
Co-I DiSanti’s research emphasizes the chemistry of volatile oxidized carbon in comets, in particular the efficiency of converting CO to H2CO and CH3OH on the surfaces of icy interstellar grains through H-atom addition reactions prior to their incorporation into the nucleus. Laboratory experiments have shown this process to be dependent both on temperatures and H-atom densities in the pre-cometary (interstellar cloud) environment. We see considerable variation among comets, both in abundances of CO, H2CO, and CH3OH (Fig. 1A) and in CO conversion efficiency (Fig. 1B). Such measurements are important for establishing the role of comets in delivering water to Earth along with the seed organic molecules from which life emerged. Our recent observations continue to support compositional diversity among comets (Mumma and Charnley 2011, DiSanti and Mumma 2008).
Figure 1. A. Abundances (relative to H2O) of oxidized carbon in several comets. Error bars show 1σ uncertainties, and upper limits ( ) are 3σ. The small beams afforded by modern IR spectrometers favor detection of native ices. B. Corresponding CO conversion efficiencies, expressible as the ratio ([H2CO]+[CH3OH])/([CO]+[H2CO]+[CH3OH]), where [] indicates mixing ratio. This approach assumes that formaldehyde and methanol are produced solely from CO, and that they are the only products. It does not include potential loss of CO (e.g., in the subsequent proto-solar environment) or its incorporation into more complex entities (e.g., polymers such as POM).
Observations during the past year
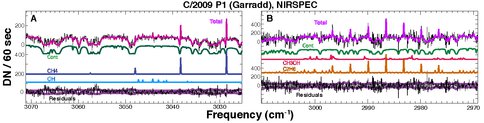
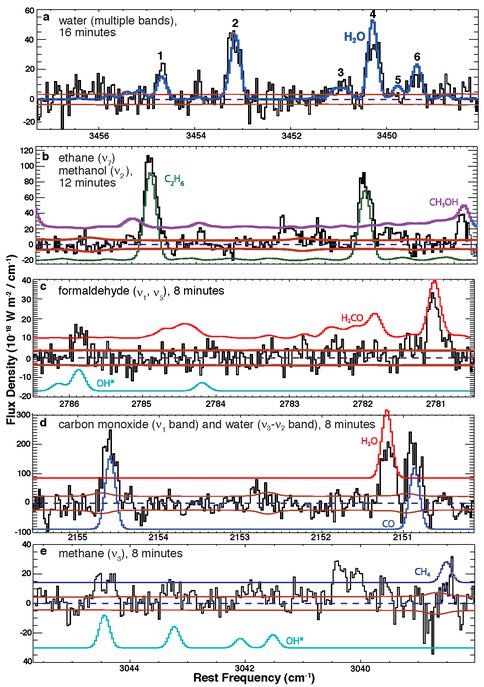
C/2009 P1 (Garradd): Our observations of comets over the past year were centered primarily on long-period comet Garradd, which reached perihelion on 2011 December 23. Using three ground-based telescope/instrument combinations (VLT/CRIRES, Keck/NIRSPEC, IRTF/CSHELL) produced two published papers featuring pre-perihelion results from August and September 2011 (Paganini et al. 2012, Villanueva et al. 2012). An additional paper (led by Co-I DiSanti) combines pre- and post-perihelion observations with NIRSPEC (Fig. 2), and is nearing submission for publication.
Figure 2. Molecular detections in long-period Comet 2009 P1 (Garradd) at heliocentric R = 1.84 AU. Modeled emissions from contributing molecules and the dust continuum are shown. “Total” refers to the sum of modeled contributions, while “Residuals” has all of these subtracted from the observed spectrum (black trace at the top of each panel).
Recent results have suggested the importance of oxidation reactions in addition to reduction (H-atom addition) in explaining observed abundances of ices in comets. Finding abundant CO2 (much higher than CO) in a substantial number of comets surveyed by the Japanese Akari space craft (Ootsubo et al. 2012, ApJ 752:15) argues for oxidation, on interstellar grains and/or following vaporization in the proto-planetary nebula. Comet Garradd had abundant CO (~ 10% relative to H2O), as first observed by us near R = 2.1 AU using CSHELL (together with NIRSPEC observations on the following day; Villanueva et al. 2012). Together with its strongly depleted C2H2 (~ 0.06%) yet nearly “normal”, this also suggests a potential role for oxidation in additional to reduction on its pre-cometary grains (neither C2H6 nor CH3OH are produced efficiently by gas-phase chemistry). Furthermore, spectra of Garradd from the DIXI space craft suggest a non-negligible abundance of CO2 in Garradd, perhaps rivaling that of CO (Feaga et al. 2012, DPS poster 313.08).
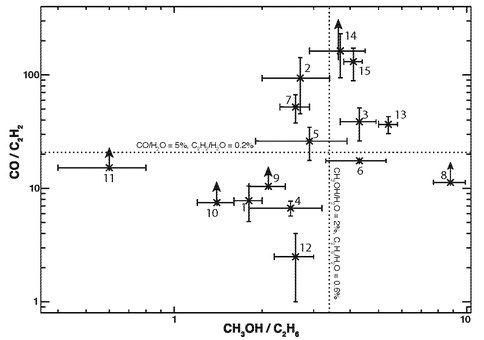
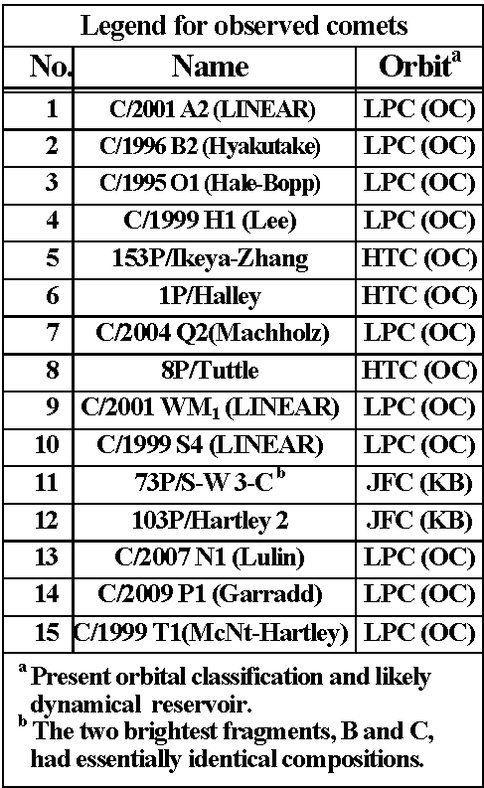
Figure 3. A comparison of measured abundance ratios (CO / C2H2) versus that of their respective hydrogenation products (CH3OH / C2H6) for 15 comets. The horizontal dotted line pertains to (approximate) median CO and “normal” C2H2, and the vertical dotted line is for “normal” CH3OH and C2H6. Points above the horizontal line but near the vertical line may indicate a more important role of oxygen reactions on the interstellar grains incorporated into the nuclei of these comets (as numbered in the right-hand table).
Fig. 3 provides a comparison of ratios of measured abundance ratios (relative to H2O) for “reactants” (CO and C2H2, both condensed onto interstellar grains) versus “products” (C2H6 and CH3OH) among a number of comets in our database. Comets with high CO/C2H2 lie well above the horizontal dotted line; this is the case with Garradd, which may represent the best case to date supporting the importance of oxidation on its pre-cometary grains.
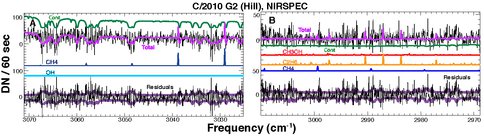
Additional comets within the past year. We observed the relatively weak long-period Comet Hill with NIRSPEC (Fig. 4), supporting our program of extending observations to comets at larger Rh (> 2 AU). These observations demonstrate the power of NIRSPEC in studying hyper-volatiles in relatively faint comets at larger R. Together with C/2009 P1 (Garradd), C/2006 W3 (Christensen), and 29P/Schwassmann-Wachmann 1, Comet Hill brings our current total of such comets to four; papers on Garradd (DiSanti et al.), Christensen (Bonev et al.), and S-W 1 (Paganini et al.) are currently in preparation, with submission targeted for fall 2012.
Figure 4. Same as Figure 2, but for faint C/2010 G2 (Hill) at R = 2.06 AU. These spectral extracts revealed emissions from CH4 and C2H6, while less volatile species CH3OH and H2O (as measured through OH) appear absent or at least very weak. This shows the power of NIRSPEC, even with relatively little on-source integration time (panel A, 40 minutes; panel B, 20 minutes).
Recent Progress on Fluorescence Modeling
To interpret our spectroscopic observations of comets we rely heavily on accurate models of predicted line-by-line intensities. The current progress period has featured significant advances in this capacity, including both quantum mechanical and empirical approaches. Rigorous quantum mechanical models for C2H6 (ν7 band), H2O (multiple bands), and CH3OH (ν3 band) were led by scientific collaborator Geronimo Villanueva. Co-I DiSanti led development of an empirical fluorescence model for the ν2 band of CH3OH (see below).
Progress on studies prior to September 2011, in which Co-I DiSanti played major roles
➢ Comet 21P/Giacobini-Zinner. In June 2005, we obtained spectra in one NIRSPEC setting that simultaneously measured H2O, C2H6 and CH3OH. As of September 1, a paper is in revision that reports abundances for these primary volatiles (DiSanti et al. 2012). The principal elements of this paper are:
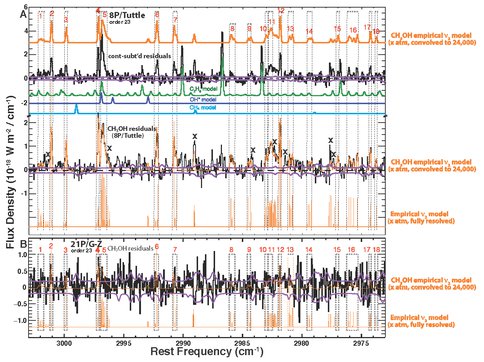
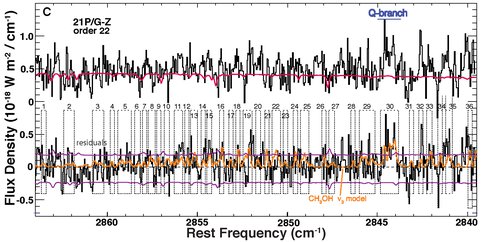
* An extremely precise and accurate production rate for H2O, incorporating analyses of rotational and spin temperatures, which were highly correlated in 21P/G-Z; this is required for obtaining robust abundances of C2H6 and CH3OH. * Confirmation of highly depleted C2H6 in 21P/G-Z, as suggested from observations during its 1998 using IRTF/CSHELL. * Development and application of an empirical model for CH3OH, including 157 prominent lines of the ν2 band. Fluorescence g-factors were established using NIRSPEC spectra of 8P/Tuttle (Fig. 5A; see also Figs. 1c and 1d in Bonev et al. 2008 ApJ 680:L61-L64). These empirical g-factors were then applied to the short-period (Jupiter Family) comet 21P/Giacobini-Zinner (Fig. 5B). This yields a production rate for CH3OH consistent with that we measured by applying our recently published fluorescence model (Villanueva et al. 2012) to ν3 band emissions in an adjacent order (Fig. 5C).
Figure 5. A. Comet 8P/Tuttle. Top traces: Continuum-subtracted residuals along with our convolved empirical fluorescence model. Subtracting modeled emissions for C2H6, OH*, and CH4 isolates emissions dominated by ν2 lines (labeled “CH3OH residuals”, lower trace), with our model superimposed. We identified 18 spectral intervals for sampling ν2 emissions. Regions marked ‘x’ contain excess intensity, perhaps due to unaccounted-for CH3OH ν9 emissions; these are omitted from our analysis. The fully-resolved model (“stick spectrum”) is shown below. B. 21P/G-Z. Application of our empirical model to its CH3OH residuals.
➢ C/2007 N3 (Lulin). We observed this long-period comet in January/February 2009 with NIRSPEC, has come to fruition in the form of a paper led by scientific collaborator Erika Gibb (Gibb et al. 2012). Co-I DiSanti led analyses of echelle orders targeting CO and H2CO.
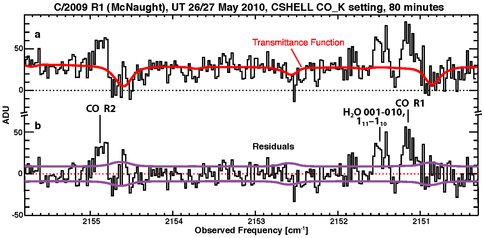
➢ C/2009 R1 (McNaught). We observed this long-period comet with IRTF/CSHELL in May and June 2010, when it was near Rh = 1.0 (Fig. 6) and 0.5 AU (Fig. 7), respectively. These data formed the basis for the research project of Undergraduate Research Associate Maria Sofia Stenborg (U. Maryland), who was mentored by Co-I DiSanti (see and text below, under “E/PO activities”).
Figure 5C. Top: Extracted spectrum for order 22, showing emissions from the n3 band of CH3OH in Comet 21P/G-Z, with the Q-branch indicated. Bottom: Continuum-subtracted residuals with (convolved) quantum band model superimposed. We measured a rotational temperature Trot = 48+10/-7 K, consistent with that we found from simultaneously observed H2O (51±3 K).
Figure 6. (a) Heavy black trace: Spectral extract showing the signal summed over 15 rows (3 arc-seconds) centered on the row having the peak emission intensity, showing combined signal in the CO_K setting on 26 and 27 May 2010. Red trace: Modeled transmittance function convolved to the spectral resolving power of the comet observations. (b) Net residual emission spectrum, obtained by subtracting the calculated transmittance function from the extracted spectrum in panel a. Rotational designations are indicated for the two encompassed CO lines. The ±1σ stochastic noise envelope is also shown, here and in the residual comet spectra in Fig. 7. One count (Analog-to-Digital Number, ADU) as plotted here corresponds to a flux density of approximately 6.3 × 10-19 W m-2 (cm-1)-1.
Figure 7. (a-e) Summary of spectral extracts for C/2009 R1 (McNaught), obtained with CSHELL on UT 22 June 2010. For each spectrum, the flux density indicates signal contained within 2×3 arc-second aperture centered on the peak molecular emission intensity. (f) Excitation plot showing the nucleus-centered H2O production rates for each of the spectral intervals in panel a, yielding a rotational temperature Trot = 101+7/-6 K.
➢ C/2007 W1 (Boattini). We measured this long-period comet in 2008 with CRIRES and NIRSPEC. The NIRSPEC results are published (Villanueva et al. 2011a), and these suggest at least two phases of ice, one polar and one apolar. The CRIRES results were obtained over a series of heliocentric distances, and a paper (led by Co-I DiSanti) is in preparation. A new fluorescence model for HCN was applied to Boattini, 8P/Tuttle, and C/2008 Q3 (Garradd), forms a manuscript that is nearing acceptance for publication (M. Lippi et. al).
E/PO activities:
Summer 2012: Co-I DiSanti served as mentor to GCA-NAI Undergraduate Research Associate Maria Sofia Stenborg (U. Maryland). Ms Stenborg processed CSHELL infrared spectra of long-period comet C/2009 R1 (McNaught). Six primary volatiles (H2O, CO, H2CO, CH3OH, C2H6, CH4) were measured near heliocentric distance 0.5 AU, and H2O and CO were measured near 1.0 AU. These observations, together with sub-millimeter-wavelength measurements processed by fellow Intern Lillian Haynes (mentored by GSFC civil servant Dr. Stefanie Milam), comprise a manuscript led by Dr. Milam, with submission to The Astrophysical Journal planned for Fall 2012.
Summer 2012: Co-I DiSanti is serving as mentor to graduate student Adam McKay (New Mexico State U.), who spent ten weeks in residence at GSFC processing pre- and post-perihelion spectra acquired on long-period Comet C/2009 P1 (Garradd), using CSHELL. Mr. McKay was awarded a NASA Graduate Student Researchers Program fellowship, with his second of three years beginning 2012 August 1. His thesis uses optical and infrared spectra of comets to constrain the relative contributions from H2O and other molecules (principally CO, CO2) to atomic oxygen, as observed through its forbidden lines at 5577 and 6300, 6364 A. Mr. McKay’s support is for 3 years at no cost to the NAI. His work supports our effort of building a compositional taxonomy of comets.
Publications
-
Gibb, E. L., Bonev, B. P., Villanueva, G., DiSanti, M. A., Mumma, M. J., Sudholt, E., & Radeva, Y. (2012). CHEMICAL COMPOSITION OF COMET C/2007 N3 (LULIN): ANOTHER ‘‘ATYPICAL’’ COMET. The Astrophysical Journal, 750(2), 102. doi:10.1088/0004-637x/750/2/102
-
Paganini, L., Mumma, M. J., Bonev, B. P., Villanueva, G. L., DiSanti, M. A., Keane, J. V., & Meech, K. J. (2012). The formation heritage of Jupiter Family Comet 10P/Tempel 2 as revealed by infrared spectroscopy. Icarus, 218(1), 644–653. doi:10.1016/j.icarus.2012.01.004
-
Paganini, L., Mumma, M. J., Villanueva, G. L., DiSanti, M. A., Bonev, B. P., Lippi, M., & Boehnhardt, H. (2012). THE CHEMICAL COMPOSITION OF CO-RICH COMET C/2009 P1 (GARRADD) AT R h = 2.4 and 2.0 AU BEFORE PERIHELION. The Astrophysical Journal, 748(1), L13. doi:10.1088/2041-8205/748/1/l13
-
Villanueva, G. L., DiSanti, M. A., Mumma, M. J., & Xu, L-H. (2012). A QUANTUM BAND MODEL OF THE ν 3 FUNDAMENTAL OF METHANOL (CH 3 OH) AND ITS APPLICATION TO FLUORESCENCE SPECTRA OF COMETS. The Astrophysical Journal, 747(1), 37. doi:10.1088/0004-637x/747/1/37
-
Villanueva, G. L., Mumma, M. J., Bonev, B. P., Novak, R. E., Barber, R. J., & DiSanti, M. A. (2012). Water in planetary and cometary atmospheres: H2O/HDO transmittance and fluorescence models. Journal of Quantitative Spectroscopy and Radiative Transfer, 113(3), 202–220. doi:10.1016/j.jqsrt.2011.11.001
-
Villanueva, G. L., Mumma, M. J., DiSanti, M. A., Bonev, B. P., Gibb, E. L., Magee-Sauer, K., … Salyk, C. (2011). The molecular composition of Comet C/2007 W1 (Boattini): Evidence of a peculiar outgassing and a rich chemistry. Icarus, 216(1), 227–240. doi:10.1016/j.icarus.2011.08.024
-
Villanueva, G. L., Mumma, M. J., DiSanti, M. A., Bonev, B. P., Paganini, L., & Blake, G. A. (2012). A multi-instrument study of Comet C/2009 P1 (Garradd) at 2.1AU (pre-perihelion) from the Sun. Icarus, 220(1), 291–295. doi:10.1016/j.icarus.2012.03.027
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Michael DiSanti
Co-Investigator
-
RELATED OBJECTIVES:
Objective 3.1
Sources of prebiotic materials and catalysts
Objective 3.2
Origins and evolution of functional biomolecules