2012 Annual Science Report
 NASA Goddard Space Flight Center
Reporting | SEP 2011 – AUG 2012
NASA Goddard Space Flight Center
Reporting | SEP 2011 – AUG 2012
Advancing Techniques for in Situ Analysis of Complex Organics
Project Summary
The overall objective of the line of work associated with technique and protocol development using laser mass spectrometry (MS) is to develop protocols for analysis of complex, nonvolatile organic molecules, such as those that might be found at Mars, Titan, comets, and other planetary bodies, with limited chemical sample manipulation, preparation, processing (as may be required by flight missions). The GCA laser MS effort is complementary to both (i) instrument development work supported by NASA programs such as ASTID, to forward the design and testing of new prototype spaceflight hardware, and (ii) ongoing research and development within Theme 4 of the GCA, concerning analytical chemical sample analysis as well as across GCA (particularly with Theme 3) to define combined analysis techniques that may affect future mission design. There are additionally aspects of this effort that relate to understanding synthetic pathways for certain complex organics in planetary environments.
Project Progress
Note: This report covers the period July 2011 – June 2012, Goddard Center for Astrobiology (GCA) Theme 4 work using laser time-of-flight mass spectrometry (TOF-MS) techniques included activities under CAN-5 (started FY09).
1. Objectives
The overall objective of the line of work associated with technique and protocol development using laser mass spectrometry (MS) is to develop protocols for analysis of complex, nonvolatile organic molecules, such as those that might be found at Mars, Titan, comets, and other planetary bodies, with limited chemical sample manipulation, preparation, processing (as may be required by flight missions). The GCA laser MS effort is complementary to both (i) instrument development work supported by NASA programs such as ASTID, to forward the design and testing of new prototype spaceflight hardware, and (ii) ongoing research and development within Theme 4 of the GCA, concerning analytical chemical sample analysis as well as across GCA (particularly with Theme 3) to define combined analysis techniques that may affect future mission design. There are additionally aspects of this effort that relate to understanding synthetic pathways for certain complex organics in planetary environments. Areas of activity with GCA support during this period included: * Support of the integration of a laser TOF-MS and an IR point spectrometer * Studies of natural analogs with reversible polarity MS * Development of protocols for pulsed ion gating and tandem MS
2. Summary of Progress
2.1. Support of the integration of a laser TOF-MS and an IR point spectrometer
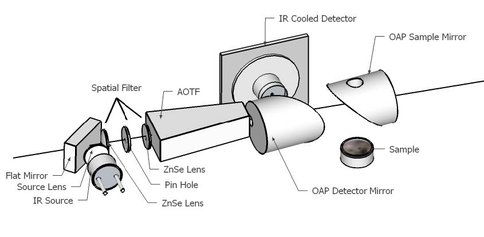
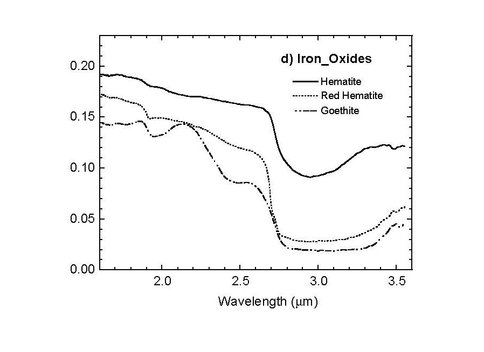
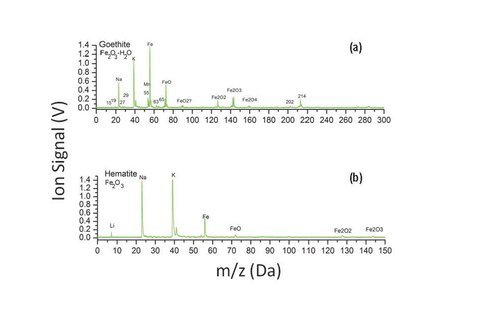
In collaboration with investigators at New Mexico State University, we have been developing a combined laser TOF-MS and IR point spectrometer under ASTID (Nancy Chanover, NMSU PI). The laser TOF-MS is based on our reversible-polarity UV laser TOF design, also supported under a separate ASTID. The near-infrared point spectrometer is based on acousto-optic tunable filter (AOTF) technology. It operates over the range 1.6 – 3.6 microns, enabling a range of rapid mineralogical and organic analyses that complement the molecular analysis of the same sample by laser TOF, which would notionally follow the AOTF in an in situ protocol (Chanover et al. 2012). The AOTF component-level design is shown in Fig. 1. Two parabolic mirrors are used to deliver tunable IR illumination and collect reflected light from a small solid sample FOV of a few mm diameter. The mirror just above the sample is bored out to accept the vertical ion inlet lens assembly of the TOF-MS. This arrangement enables the laser to probe the composition of the same spot as the AOTF spectrometer. During this year the final miniature AOTF was fabricated, tested, and delivered to Goddard where it was integrated with the TOF-MS. NAI support has been key to the level of examination of analog samples going on in parallel with, and guiding the development of, the instrument prototype. As one example, the analysis of iron oxide minerals, goethite and hematite, is important for their differing formation processes on Earth and levels of hydration in the mineral matrix. These minerals are also associated with different (bio)organic preservational environments and are examples of phases that would benefit the assessment of Mars habitability if they could be distinguished at the fine spatial scales of these spectrometers. Prototype AOTF spectra (Fig. 2) of goethite showed spectral signatures of the –OH stretch indicating enhanced hydration relative to the unhydrated mineral hematite. In comparison, the TOF-MS spectra (Fig. 3) reveal composition of the mineral, showing clear iron and iron oxide species in the mass spectrum, with higher oxide abundances in the hydrated goethite as expected. In a series of spectra by both techniques, it may be possible to calibrate the hydration state and ultimately use the data in real-time to identify particular organic and mineralogical “zones” in a layered or otherwise heterogeneous sample.
Fig. 1 Schematic layout of AOTF point spectrometer components. Courtesy of D. Glenar, New Mexico State University.
Fig. 2 Sample spectra of hematite and goethite acquired with the AOTF point IR spectrometer. Courtesy of N. Chanover, New Mexico State University.
Fig. 3 Sample spectra of goethite (top) and hematite (bottom) acquired with the laser TOF-MS. These measurements of a set of mineral samples reveal composition and provide information about the degree of mineral hydration, such as for the iron oxide family of minerals.
2.2. Studies of natural analogs with reversible polarity MS
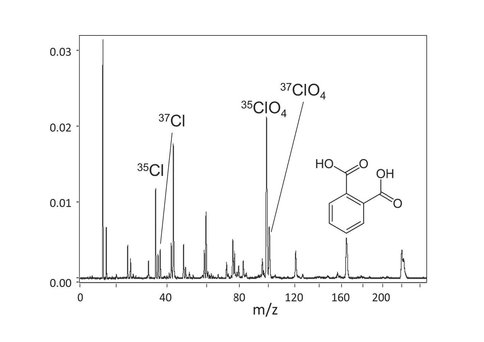
We have continued the development of techniques and methods associated with combined positive and negative ion mass spectrometry of planetary analogs, using our newest reversible polarity (RP) TOF instrument and associated reference systems including collaboration with the Theme 3 Astrobiology Analytical Laboratory (M. Callahan, J. Dworkin, J. Elsila). Previously reported analyses of Mars regolith analogs spiked with perchlorates, as discovered by the Phoenix lander, have been further developed by mixing both sodium perchlorate and a model carboxylic acid, phthalic acid, into a kaolinite clay matrix, which could be a model for near-surface phases at the base of Mt. Sharp in Gale crater. We show in Fig. 4 that both the ClO4 anion and the phthalic acid molecular ion are observed in negative mode of the RP-TOF. This is a significant finding that suggests that the heating effects in laser desorption/ionization are negligible. Some solid regolith analytical techniques that use sustained heating to volatilize sample constituents also may promote the oxidation of organic species that may be co-located with perchlorate salts on Mars. It is clear from the simultaneous appearance of the perchlorate anion and the phthalic acid molecular ion that laser TOF-MS, in contrast does not appear to impart sufficient heat to the sample to induce oxidation of this organic species.
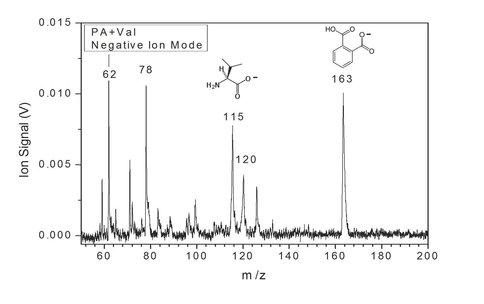
In a separate example we prepared a mixture of two organic species from different classes, the amino acid valine (Val) and the carboxylic acid phthalic acid (PA). The two compounds were mixed and deposited onto a sample probe for RP-TOF measurements, and the mass spectrum is shown in Fig. 5. The importance of negative ion detection is especially evident in this sample. In positive ion mode, neither valine nor phthalic acid is observed at the parent masses. It is only in negative mode that the intact molecular ions can be clearly detected.
Fig. 4 Simultaneous detection of perchlorate signatures and the molecular ion of phthalic acid shows that organics co-located with perchlorate salts on Mars may be detectable with the reversible polarity TOF-MS.
Fig. 5 In a mixture of an amino acid, valine, and a carboxylic acid, phthalic acid, the parent ions are only detectable in negative ion mode, highlighting the importance of reversible polarity in laser desorption/ionization mass spectrometry.
2.3. Development of protocols for pulsed ion gating and tandem MS
We have continued working on laboratory tests and protocol development in support of RP-TOF development objectives including an ion gating capability for improvements to instrument mass precision and sensitivity. The ion gate works by deflecting selected masses before they reach the detector. As a result, the mass spectrum can be simplified by rejecting dominant low-mass salt peaks that produce detector noise, or a single mass can be isolated, sometimes with post-source fragments of an organic parent mass to elucidate peak identification. We have developed and tested a pulsed pin gate (PPG) using a single high-gauge wire the receives a pulsed voltage to generate fast switching between the on and off states. The key to the PPG is to position the wire between two grid electrodes that preserve the desired field profile on either side of the gate pin. These grids define the length of the gate along the ion flight axis, and for a given ion energy, the distance between them determines how finely in time the masses are discriminated. A recent demonstration of selective gating of a calibration compound, tributyl phosphate, is shown in Fig. 6. By adjusting the ion gate timing, the predicted parent masses reveal the selective isolation of the parent mass at m/z 268. A particular benefit of ion gating is the enhancement of signatures of post-source fragmentation, as is seen for sequential loss of the three butyl groups for this molecular structure. Post-source decay occurs after the ionization process, and as a result, the fragments are grouped in energy with their respective parent molecules and can be selected with a single gating pulse for clarity of data interpretation. This kind of analytical tool becomes of significant importance when dealing with a complex mixture and implies that a pulsed pin gate could be scanned over the TOF (one axis of fragmentation information) as well as a function of the desorption/ionization energy (a second axis) yielding a 2D “fragment” space without necessarily introducing gases to induce collisions in the selected parent ion population while drifting through the instrument (a more “classic” approach to MS/MS in a TOF instrument). A new PIDDP development in the areas of two-step laser ionization and pulsed ion gating was selected for award (PI: Stephanie Getty) based partly on these promising results supported by NAI research.
Fig. 6 The pulsed pin gate has been demonstrated for a calibration compound, tributyl phosphate. The sequential loss of each of the three butyl groups for this structure is evident in the appearance of three evenly spaced decay product peaks.
Publications
-
Li, X., Brinckerhoff, W. B., Managadze, G. G., Pugel, D. E., Corrigan, C. M., & Doty, J. H. (2012). Laser ablation mass spectrometer (LAMS) as a standoff analyzer in space missions for airless bodies. International Journal of Mass Spectrometry, 323-324, 63–67. doi:10.1016/j.ijms.2012.06.020
-
Bishop, J. L., Franz, H. B., Goetz, W., Blake, D. F., Freissinet, C., Steininger, H., … Darby Dyar, M. (2013). Coordinated analyses of Antarctic sediments as Mars analog materials using reflectance spectroscopy and current flight-like instruments for CheMin, SAM and MOMA. Icarus, 224(2), 309–325. doi:10.1016/j.icarus.2012.05.014
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
William Brinckerhoff
Co-Investigator
Ricardo Arevalo Jr.
Collaborator
Melissa Floyd
Collaborator
Stephanie Getty
Collaborator
-
RELATED OBJECTIVES: