2010 Annual Science Report
 VPL at University of Washington
Reporting | SEP 2009 – AUG 2010
VPL at University of Washington
Reporting | SEP 2009 – AUG 2010
Understanding the Early Mars Environment
Project Summary
In 2009-2010, VPL’s investigations into Mars were carried out in two major themes: investigation of the how the climate and chemistry of early Mars might (or might not) allow liquid water at the surface, and follow up science to the surprising discovery of perchlorate by NASA’s 2008 Phoenix Lander. VPL determined that, contrary to previous thought, SO2 could not keep early Mars warm, due to the inevitable formation of sulfate aerosols which counteract any warming due to SO2. Investigations into the formation of perchlorate in Earth’s deserts provide clues towards potential formation of Martian perchlorate, and specific predictions were made to all for future rovers to discriminate between evaporated versus frozen perchlorate minerals.
Project Progress
In 2007, Halevy et al. suggested in Science that SO2 may have been the key to warming early Mars. They suggested a feedback loop similar to that proposed for CO2 cycling on Earth: If the Martian climate was too cold, volcanic SO2 would have built up in the atmosphere, creating additional greenhouse effect, and thereby warming the surface. They did not, however, perform any calculations to test this prediction.
We first performed 1-D climate model simulations where we added mixtures of SO2 and CO2 into the early Martian atmosphere. We found that SO2 mixing ratios in excess of 10 ppmv, combined with multiple bars of CO2 could theoretically warm up early Mars above the freezing point. However, these calculations did not take into account what would happen to SO2 photochemically in the early Martian atmosphere.
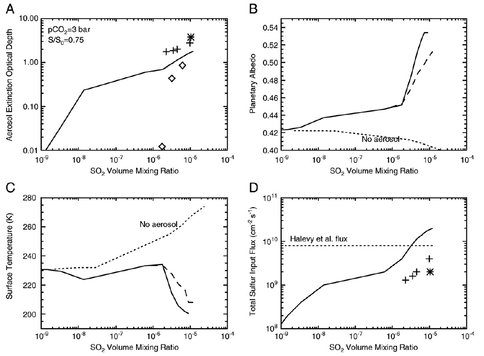
SO2 in Earth’s atmosphere is rapidly oxidized to H2SO4, sulfuric acid, which then condenses out to form sulfate aerosols, which both reflect and scatter light. VPL showed that the same thing happens, albeit not quite as fast, in a reduced early Martian atmosphere. Results for a representative 3-bar CO2-SO2-H2O atmosphere (calculated with our updated 1-D photochemical model) are shown in the figure. Panel 'A’ shows aerosol extinction optical depth as a function of SO2 mixing ratio. Optical depths become large, i.e., >1, for SO2 concentrations exceeding a few ppmv. Panel 'B’ shows the effect of the aerosols on planetary albedo. The albedo increases rapidly at SO2 concentrations exceeding a few ppmv. Panel 'C’ shows calculated mean surface temperatures for a 'no aerosol’ case compared with two cases in which aerosols are included. The ‘no aerosol’ case warms monotonically with increasing SO2, whereas the two 'aerosol included’ cases both decrease with increasing SO2 because the increase in planetary albedo outweighs the increase in the greenhouse effect. Panel 'D’ shows the calculated total sulfur (SO2 + H2S) outgassing rates in our model compared to the outgassing rate assumed by Halevy et al. We conclude that, if early Mars was indeed warm, it must have been a result of factors other than SO2 (Tian et al 2010).
Panel 'A’-aerosol extinction optical depth at 5500 Å. The solid curve is for sulfate aerosols under normal (terrestrial) rainout conditions. The diamond symbols are for S8 aerosols under normal rainout condition. The crosses are for sulfate aerosols for rainout reduced by a factor of 100. The star is for sulfate aerosols for rainout reduced by a factor of 1000. Panel 'B’-planetary albedo. Panel 'C’-surface temperature. Panel 'D’-combined (SO2+H2S) fluxes, with the same symbols as those in panel A. These calculations are for a 3-bar atmosphere with S/S0 = 0.75. Dotted curves in panels B and C show similar calculations in which sulfate aerosols are neglected
In addition, VPL ran energy balance models to simulate the effects of surface temperature on putative Martian rainout. This can serve as a stabilizing feedback to maintain “cool” temperatures at the boundary between evaporation-driven and sublimation-driven hydrological cycles. This work in progress was presented by undergraduate Jonathan Briener at the AGU Fall meeting. (AGU Fall Meeting p51D-1161, 2009)
NASA’s announcement of the discovery of perchlorate on Mars came fortuitously with the announcement of our DDF award. As it became clear very quickly that the SO2 was an unambiguous null result, we were able to repurpose the time of senior personnel from their detailed follow-on work to address perchlorate. Co-I’s Catling, Claire, and Zahnle enhanced their photochemical model and provided the first explanation of gas-phase atmospheric production of perchlorate over Earth’s Atacama Desert (Catling et al. 2010). This “value-added” DDF project enabled VPL Co-I’s to successfully propose to the Mars Fundamental Research program to model the formation of perchlorate on Mars. In a second re-purposing, Co-I Marion added perchlorate species into the FREZCHEM model, instead of sulfides as originally proposed. We show that calcium perchlorate would not have precipitated from a mixture of water added to Phoenix soil, and that different ratios of sodium and magnesium perchlorate would result from long-scale slow evaporative process versus a more rapid freezing pathway. These results suggest that future sample return or in-sity X-ray diffraction may help discriminate between evaporation and freezing processes in the Martian soil (Marion et al. 2010).
Publications
-
Catling, D. C., Claire, M. W., Zahnle, K. J., Quinn, R. C., Clark, B. C., Hecht, M. H., & Kounaves, S. (2010). Atmospheric origins of perchlorate on Mars and in the Atacama. Journal of Geophysical Research, 115. doi:10.1029/2009je003425
-
Marion, G. M., Catling, D. C., Zahnle, K. J., & Claire, M. W. (2010). Modeling aqueous perchlorate chemistries with applications to Mars. Icarus, 207(2), 675–685. doi:10.1016/j.icarus.2009.12.003
-
Tian, F., Claire, M. W., Haqq-Misra, J. D., Smith, M., Crisp, D. C., Catling, D., … Kasting, J. F. (2010). Photochemical and climate consequences of sulfur outgassing on early Mars. Earth and Planetary Science Letters, 295(3-4), 412–418. doi:10.1016/j.epsl.2010.04.016
- Breiner, J., Domagal-Goldman, S. & Claire, M.W. (2009). Modeling rainout on ancient Mars as a function of atmospheric forcings. AGU Fall Meeting. San Francisco, CA.
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
David Catling
Co-Investigator
Pamela Conrad
Co-Investigator
David Crisp
Co-Investigator
James Kasting
Co-Investigator
Giles Marion
Co-Investigator
Victoria Meadows
Co-Investigator
Kevin Zahnle
Co-Investigator
David Des Marais
Collaborator
Conway Leovy
Collaborator
Dave Pollard
Collaborator
Shawn Domagal-Goldman
Postdoc
Jacob Haqq-Misra
Graduate Student
Tyler Robinson
Graduate Student
Jonathan Breiner
Undergraduate Student
Megan Smith
Undergraduate Student
-
RELATED OBJECTIVES:
Objective 1.1
Formation and evolution of habitable planets.
Objective 2.1
Mars exploration.