2010 Annual Science Report
 VPL at University of Washington
Reporting | SEP 2009 – AUG 2010
VPL at University of Washington
Reporting | SEP 2009 – AUG 2010
Thermodynamic Efficiency of Electron-Transfer Reactions in the Chlorophyll D-Containing Cyanobacterium, Acharyochloris Marina
Project Summary
Photosynthesis produces planetary-scale biosignatures – atmospheric oxygen and the color of photosynthetic pigments. It is expected to be successful on habitable extrasolar planets as well, due to the ubiquity of starlight as an energy source. How might photosynthetic pigments adapt to alternative environments? Could oxygenic photosynthesis occur at much longer wavelengths than the red? This project is approaching these questions by using a laser technique to study the recently discovered cyanobacterium, Acaryochloris marina, which uses the chlorophyll d pigment to perform its photosynthesis at wavelengths longer than those used by the much more prevalent chlorophyll a. Whether A. marina is operating more efficiently or less than Chl a-utilizing organisms will indicate what wavelengths are the ultimate limit for oxygenic photosynthesis.
Project Progress
Thermodynamic efficiency of electron-transfer reactions in purified Photosystem I and II complexes in the Chlorophyll d-containing cyanobacterium, Acaryochloris marina
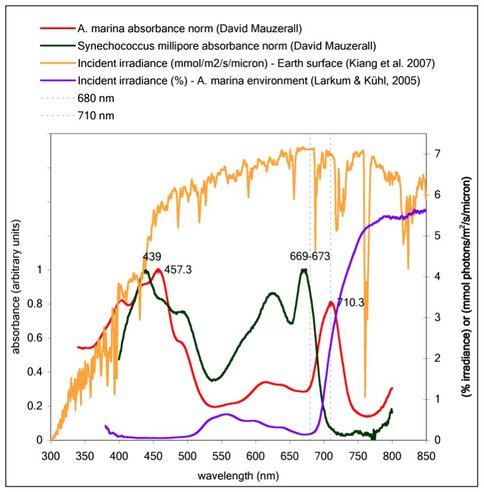
Under funding from the NAI Director’s Discretionary Fund (DDF) 2009, we have been continuing measurements the efficiency of photon energy storage by wavelength in whole intact cells of the cyanobacterium Acaryochloris marina. A. marina is the only known organism to have chlorophyll d (Chl d) to use photons at wavelengths, in prior literature, in the far-red (713-715 nm) and near-infrared (740 m), whereas all other oxygenic photosynthetic organisms use chlorophyll a (Chl a) with absorbance peaks at 680 nm and 700 nm. After some technical issues were overcome in the first year and stability of our laser was achieved, we have obtained measurements indicating that A. marina is in fact exhibiting efficiencies higher than or comparable to that in Chl a-utilizing organisms. These results imply that oxygenic photosynthesis can operate quite effectively at far-red/near-infrared wavelengths and is not suffering from losses to back reactions, something that had been unknown up till now. This then extends what is plausible on extrasolar planets, and in light of the discovery just this year of chlorophyll f, which is further red-shifted by ~6 nm, it appears that oxygenic photosynthesis may be possible at yet longer wavelengths. Also, our spectral efficiency measurements provide direct, credible evidence for the wavelengths of peak absorbance of the reaction centers of A. marina, which we find to be slightly blue-shifted from indirect attempts in the literature, our findings being at 710 nm for PS II and 730 nm for PS I.
The above measurements on whole cells have largely been completed and a paper is in preparation. Measurements with an inhibitor of Photosystem II (PS II) will allow us one approach to distinguishing the separate contributions of PS I and PS II to the overall efficiency. Extraction of purified complexes of Photosystem I and II commenced this past year in the lab of Co-I Blankenship, and measurements of their individual efficiencies will be done in the coming year. For purification of these complexes, Dr. Min Chen at the University of Sydney, NSW, Australia, has also been brought on as Co-Investigator for her expertise with A. marina. Photosystem I and Photosytem II complexes of A. marina have been prepared by extracting purified membranes with 1% dodecyl maltoside detergent, followed by sucrose density gradient centrifugation and ion exchange chromatography. The complexes are spectrally similar to those prepared by previous researchers. Further analysis is underway.
Complementing these lab studies, NAI postdoctoral fellow Steven Mielke’s has theoretically modeled redox potentials along the electron transfer pathway in Photosystem II with Chl a versus Chl d, using the Multi-Conformer Continuum Electrostatics (MCCE) computer program of collaborator Dr. Marilyn Gunner, at City College of New York. Thus far, the modeling has uncovered subtle differences in redox potentials when Chl d has replaced Chl a. More detail on this work may be found under the summary for NASA Postdoctoral Program fellow Steven Mielke.
Whole-cell absorbance spectra for Chl d-utlizing Acarychloris marina and Chl a-utlizing Synechococcus, with solar photon flux density spectrum and in situ relative spectral irradiance in natural habitat of A. marina (underneath marine ascidian).
Publications
- Mielke, S., Mauzerall, D., Blankenship, R.E. & Kiang, N.Y. (2010). Constraining photosynthetic biosignatures: Spectral photoacoustic measurements of photon energy use efficiency in the far-red/near-infrared by the chlorophyll d-utilizing cyanobacterium Acarychloris marina. International Photosynthesis Congress 2010. Beijing, China.
- Mielke, S., Mauzerall, D., Blankenship, R.E. & N.Y., K. (2010). Constraining photosynthetic biosignatures: Spectral photoacoustic measurements of photon energy use efficiency in the far-red/near-infrared by the chlorophyll d-utilizing cyanobacterium Acarychloris marina. Revisiting the Habitable Zone Workshop. Talaris Conference Center, Seattle, WA.
- Mielke, S.P., Kiang, N.Y., Blankenship, R.E. & D., M. (2010). Thermal Efficiency of Photosynthesis in the Cyanobacterium, Acaryochloris marina. Eastern Regional Photosynthesis Conference. Marine Biological Laboratory, Woods Hole, MA.
- Mielke, S.P., Kiang, N.Y., Blankenship, R.E., Gunner, M.R. & Mauzerall, D. (2010). Photosynthetic Electron-Transfer in the Cyanobacterium, Acaryochloris marina. Astrobiology Science Conference 2010 – Evolution and Life: Surviving Catastrophes and Extremes on Earth and Beyond. League City, Texas.
- Mielke, S.P., Kiang, N.Y., Gunner, M.R., Blankenship, R.E. & Mauzerall, D. (2009). Photosynthetic Electron Transfer in the Cyanobacterium, Acaryochloris marina. NAI Executive Council Meeting. Yellowstone National Park, Wyoming.
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Robert Blankenship
Co-Investigator
David Mauzerall
Co-Investigator
Marilyn Gunner
Collaborator
Steven Mielke
Postdoc
-
RELATED OBJECTIVES:
Objective 3.2
Origins and evolution of functional biomolecules
Objective 4.2
Production of complex life.
Objective 5.1
Environment-dependent, molecular evolution in microorganisms
Objective 5.3
Biochemical adaptation to extreme environments
Objective 6.2
Adaptation and evolution of life beyond Earth
Objective 7.2
Biosignatures to be sought in nearby planetary systems