2010 Annual Science Report
 University of Wisconsin
Reporting | SEP 2009 – AUG 2010
University of Wisconsin
Reporting | SEP 2009 – AUG 2010
Project 5C: Fluid-Mineral Fractionation of Mg Isotopes and Tracing the Origin of Sulfate Minerals
Project Summary
We are developing an experimental program to characterize the Mg isotope fractionation between fluids and minerals in order to use the Mg isotope system to characterize the paleoenvironmental conditions of ancient terrestrial rocks and samples from Mars. Our initial work has focused on Mg isotope fractionation between aqueous Mg and epsomite. Magnesium sulfate is present on the surface of Mars, where, for example, up to 36 wt. % sulfate has been found in some outcrops on the Martian surface, of which Mg-sulfate is the most abundant (Clark et al., 2005). Sulfates are a major water reservoir for the Martian surface and thus it is inferred that there was a period of aqueous alteration on Mars (e.g., Wang et al., 2008). Knowledge of the controls on Mg isotope fractionation in the system fluid and Mg sulfate will allow us to ultimately characterize the evaporation rates and Mg fluxes that occurred during one of the wettest periods in Mars History.
Project Progress
We employed a “three-isotope” method to evaluate the Mg isotope exchange kinetics and approach to Mg isotope equilibrium between epsomite and saturated MgSO4 solutions at 7, 20, and 40 °C. Magnesium isotope exchange was promoted by a recrystallization process, where fine-grained epsomite seed crystals were suspended in saturated MgSO4 solutions and the epsomite crystals recrystallized into coarser grains via Ostewald ripening. In these experiments the concentration of the MgSO4 solution did not change with time indicating that there was no net mass transfer of Mg.
The three-isotope method involves isotope exchange between two components, one of which is enriched in one of the isotopes. At isotopic equilibrium, the two components will lie along a secondary mass fractionation line, whose position on a δ-δ plot is dictated by the isotopic mass balance of the two components. In our experiments we used an enriched 25Mg isotope tracer, where a 25Mg-enriched MgSO4 solution was reacted with epsomite with “normal” isotope composition (A series of experiments), as well as the reverse, where the MgSO4 solution with “normal” isotope composition was reacted with 25Mg-enriched epsomite (B series of experiments). Each experiment used 10 to 20 centrifuge tubes of identically prepared mixtures of crystals and solution, where individual tubes were sacrificed and sampled for isotopic analysis for time-series sampling.
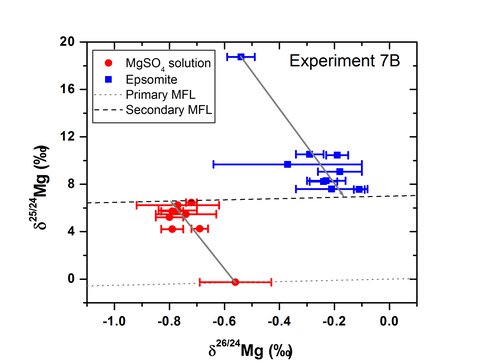
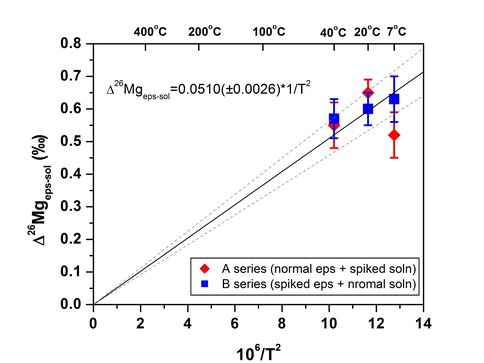
Figure 1 shows the results for an experiment conducted at 7°C. Two centrifuge tubes were sacrificed at 1, 3, 7, and 15 days providing duplicate measurements at each time point. After 15 days the aqueous Mg and epsomite each plotted on the secondary mass fractionation line indicating that complete isotope exchange had occurred between the two phases. The difference in δ26Mg between the epsomite and saturated Mg solution is interpreted to represent the equilibrium fractionation factor. The equilibrium fractionation factor measured using the A-series and B-series experiments at 7, 20, and 40°C are plotted on an Arrenhius plot in Figure 2 shows the temperature dependence of the fractionation factor is 1000lnαepsomite-solution] = 0.0510 ±0.0026×106/T2 using the measured fractionation factors and extrapolating to zero fractionation at infinite temperature. Because the temperature dependence is small (0.004 ‰/°C), the epsomite-fluid fractionation would be a poor isotope thermometer, but the magnitude of this fraction (Δ26Mgeps-sol = 0.59 ‰ at 20°C) is large enough to produce significant Mg isotope variations in evaporite sequences, depending on the degree of closed-system behavior. Magnesium isotopes, therefore, could be used as an indicator of evaporative processes.
Figure 1:. delta 25Mg vs delta 26Mg of isotope exchange experiment between epsomite and saturated Mg solution done at 7oC. The gray lines show the isotope composition at 100% isotope exchange.
Figure 2:. Arrhenius plot of measured epsomite-solution Mg isotope fractionation factor at 7, 20, and 40oC showing the temperature dependency of this fractionation factor. Each point is the average of duplicate experiments calculated by extrapolation to 100% isotope exchange. Error bars show the range in the duplicate measurements.
Publications
-
Li, W., Beard, B. L., & Johnson, C. M. (2011). Exchange and fractionation of Mg isotopes between epsomite and saturated MgSO4 solution. Geochimica et Cosmochimica Acta, 75(7), 1814–1828. doi:10.1016/j.gca.2011.01.023
-
Wang, A., Bell, J. F., Li, R., Johnson, J. R., Farrand, W. H., Cloutis, E. A., … Greenberger, R. (2008). Light-toned salty soils and coexisting Si-rich species discovered by the Mars Exploration Rover Spirit in Columbia Hills. Journal of Geophysical Research, 113(E12), None. doi:10.1029/2008je003126
- Clark, B.C., Morris, R.V., McLennan, S.M., Gellert, R., Jolliff, B., Knoll, A.H., Squyres, S.W., Lowenstein, T.K., Ming, D.W., Tosca, N.J., Yen, A., Christensen, P.R., Gorevan, S., Bruckner, J., Calvin, W., Dreibus, G., Farrand, W., Klingelhoefer, G., Waenke, H., Zipfel, J., Bell, I.J.F., Grotzinger, J., McSween, H.Y. & Rieder, R. (2005). Chemistry and mineralogy of outcrops at Meridiani Planum. Earth and Planetary Science Letters, 240(73-94).
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Max Coleman
Co-Investigator
Clark Johnson
Co-Investigator
Weiqiang Li
Postdoc
-
RELATED OBJECTIVES:
Objective 2.1
Mars exploration.
Objective 4.1
Earth's early biosphere.
Objective 7.1
Biosignatures to be sought in Solar System materials
Objective 7.2
Biosignatures to be sought in nearby planetary systems