2010 Annual Science Report
 University of Wisconsin
Reporting | SEP 2009 – AUG 2010
University of Wisconsin
Reporting | SEP 2009 – AUG 2010
Project 5B: Detection of Biosignatures in Extreme Environments, Analogs for Mars
Project Summary
The planet Mars may have been warmer in the past and at one time probably had an acidic, wet environment but currently it is cold and dry. Past conditions and maybe even present ones, although extreme, could support microbial life and we have investigated life in two extreme analog environments. The Río Tinto is an acid river is Spain where from an airborne remote survey we have monitored the progress of a metabolic process in which iron, rather than carbon, is oxidized by bacteria. At the site of a former munitions factory in Israel we have shown that bacteria can live off the chemical energy of the chemical compound perchlorate (recently found on Mars), despite adverse conditions and negligible amounts of water in the environment.
Project Progress
We have investigated two martian analogs and ironically, both are sites where anthropogenic pollution has created appropriate environments. In the Río Tinto area there are profound effects of Acid Mine Drainage and the site is a plausible analog for an early Mars acid environment (Amils et al., 2007). Weathering of the pyrite ore waste piles is mediated by iron and oxidizing bacteria, such as Acidithiobacillus ferrooxidans and Leptospirillum ferrooxidans (López-Archilla et al., 2001). Although the river is renowned for its red color from ferric iron compounds, we have also sampled the source areas, some of which are dominated by ferrous iron and have an intense green color (Hubbard et al., 2009 and Figure 1). In laboratory incubation experiments, microbes first oxidize sulfide to produce
Figure 1. We have sampled Río Tinto waters which can be green [Fe(II)] but are oxidized microbially to red [Fe(III)] solutions.
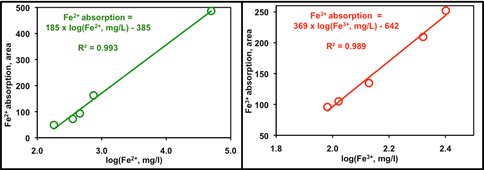
sulfate which leaves green colored ferrous iron in solution (Yu et al., 2001). The subsequent stage is microbial oxidation to produce red ferric iron from ferrous. After this stage, only abiotic sulfide oxidation by ferric iron occurs. In successive field campaigns we recorded considerable spatial and temporal variability of the green and red waters. We hypothesized that the process is periodically stimulated by introduction of oxygenated water after rainstorms. Therefore, we needed to map and record the changes from green to red but could not monitor and sample over the whole area. So we developed the novel remote sensing approach, the proof of concept of which we report here for the first time. We were able to obtain data from a remote sensing survey undertaken specifically for this purpose by the UK NERC Airborne Research and Survey Facility. We deployed a thematic mapper, a hyperspectral imager and LIDAR. We simultaneously collected samples and made ground measurements for calibration purposes. Laboratory spectral experiments revealed two absorption features in the visible and near infrared regions that together corresponded to total iron concentrations in water. However, and more importantly, the IR absorption feature represented ferrous iron thus showing the potential for using this approach for monitoring. Analysis of the remotely-acquired data showed excellent correlation with ground truthing samples (Figure 2). Although these are the first results using this approach, we have shown
Figure 2. Calibration of remote spectral data against lab. measurements of the same waters.
that we could directly monitor a microbial process, other than one that produces chlorophyll, by remote sensing.
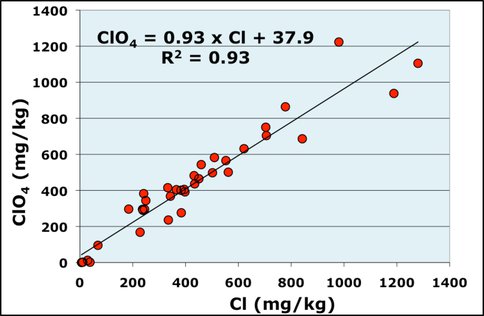
The other analog relates to the Phoenix mission in which the Wet Chemistry Laboratory instrument analyzed soil and detected perchlorate (Hecht et al., 2009), which occurs naturally on Earth but mainly is manufactured for use in munitions, fireworks and airbags in cars and the careless disposal of which can pollute drinking-water sources. This analog site is in Israel, the site of a former factory which produced and spilt large amounts of perchlorate (Gal et al., 2009). We analyzed material from various depths from the surface to 35 meters in a sampling well in the unsaturated zone beneath a former waste pond not used for 20 years. Perchlorate was made from a chloride precursor and both species occurred together, producing crude correlation of concentrations (Fig 3). We wanted to test if
Figure 3. Concentrations of perchlorate versus chloride for all samples from Ramat Hasharon area.
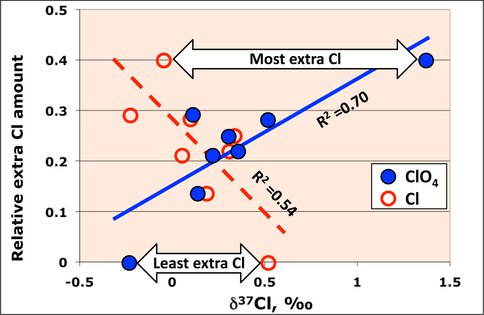
microbial perchlorate reduction might be occurring despite the fact that it is an anaerobic process and that the sediments, consisting of sands and sandy clays and occasional clay layers, were in the fully aerobic unsaturated zone. Microbial reduction of perchlorate produces a large isotope fractionation (Coleman et al., 2003) in which the δ37Cl value of the chloride produced is more negative than that of the perchlorate from which it is formed and the residual perchlorate gives successively more positive values. We made δ37Cl measurements of pairs of chloride and perchlorate samples from various depths in the well. Clearly, in the presence of a considerable amount of chloride it would be difficult to detect the relatively small additions from perchlorate reduction. We took advantage of the correlation line (Fig. 3) to identify those samples with more chloride and less perchlorate relative to the line. We then examined the isotope compositions relative to calculated excess of chloride. It can be seen that there are two relationships: increase in Extra chloride relates negative δ37Cl of chloride and positive δ37Cl of perchlorate, both indicating perchlorate reduction (Fig. 4). Subsequent microbial assays confirmed the presence of the bacteria. We
Figure 4. Chlorine isotope compositions of paired chloride and perchlorate samples from the same depth plotted against the amount of extra chloride, normalized to the sample with least Cl, relative to the best fit line (Fig. 3).
calculated that up to 10% of the perchlorate had been reduced. We hypothesize that the bacteria occupy microenvironments in a system with minimal water and organic matter but despite those conditions and being in the aerobic zone, they have grown and thrived.
Amils, R., González-Toril, E., Fernández-Remolar, D., Gómez, F., Aguilera, A., Rodríguez, N., Malki, M., García-Moyano, A., Fairén, A.G., de la Fuente, V., Sanz, J.L. (2007) Extreme environments as Mars terrestrial analogs: the Rio Tinto case. Planet. Space Sci. 55 370–381.
Coleman M, Ader M, Chaudhuri SK and Coates JD (2003) Microbial isotopic fractionation of perchlorate chlorine. Appl. Env. Microbiology 69 4997-5000.
Gal, H., Weisbrod, N., Dahan, O., Ronen, Z. and Nativ, R. (2009) Perchlorate accumulation and migration in the deep vadose zone in a semiarid region._ J. Hydrol._ 378 142-149.
Hecht MH, et al. (2009) Detection of perchlorate and the soluble chemistry of Martian soil at the Phoenix lander site. Science 325 64-67.
Hubbard CG, Black S and Coleman ML (2009) Determining sulfide oxidation mechanisms from aqueous geochemistry and oxygen isotopes in acid mine drainage from the Río Tinto, SW Spain. Chem. Geol. 265 321–334.
López-Archilla, A.I.,Marin, I., Amils, R. (2001) Microbial community composition and ecology of an acidic aquatic environment: the Tinto River, Spain. Microb. Ecol. 41, 20–35.
Yu, J.Y., McGenity, T.J., Coleman, M.L., 2001. Solution chemistry during the lag phase and exponential phase of pyrite oxidation by Thiobacillus ferrooxidans. Chem. Geol. 175 307–317.
Publications
-
Hubbard, C. G., Black, S., & Coleman, M. L. (2009). Aqueous geochemistry and oxygen isotope compositions of acid mine drainage from the Río Tinto, SW Spain, highlight inconsistencies in current models. Chemical Geology, 265(3-4), 321–334. doi:10.1016/j.chemgeo.2009.04.009
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Stuart Black
Collaborator
Ofer Dahan
Collaborator
Chris Hubbard
Collaborator
Zeev Ronen
Collaborator
Noam Weisbrod
Collaborator
Kevin White
Collaborator
-
RELATED OBJECTIVES:
Objective 2.1
Mars exploration.
Objective 7.1
Biosignatures to be sought in Solar System materials