2010 Annual Science Report
 University of Wisconsin
Reporting | SEP 2009 – AUG 2010
University of Wisconsin
Reporting | SEP 2009 – AUG 2010
Project 3A: Quantifying the Amount of Free Oxygen in the Neoarchean Photic Zone Through Combined Fe and Mo Isotopes
Project Summary
The history of Earth’s atmospheric evolution is critical for understanding the interplay between life and the physical environment, both here on Earth, and potentially on other worlds. The majority of models for Earth’s atmospheric evolution, and evidence from the geologic record, suggest that the atmosphere was virtually devoid of free oxygen through the Archean and into the earliest Proterozoic, and became more oxygen-rich over time in a punctuated fashion. The earliest significant increase in atmospheric oxygen has been termed the “Great Oxidation Event” (GOE), and is commonly considered to have occurred between ~2.4 and 2.2 Ga (Holland, 1984, 2006 and references therein). However, some geologic evidence indicates that the free oxygen content of the atmosphere-hydrosphere system may have been similar to that of the modern Earth since prior to 3.5 Ga (e.g., Hoashi et al. 2009), or that it may have had a more complex history with numerous instances of oxygen production and consumption prior to the GOE, but perhaps on a more localized scale (Anbar et al., 2007; Frei et al., 2009; Godfrey and Falkowski, 2009). Additionally, evidence from biomarkers (Brocks et al., 1999; Eigenbrode et al., 2008; Waldbauer et al., 2009) and carbon isotopes (Hayes, 1983; Eigenbrode and Freeman, 2006) suggest that both oxygen producers and consumers existed by ~2.7 Ga.
Project Progress
In Year 3, we performed a study of Fe and Mo isotope compositions of Neoarchean carbonates as old as 2.7 Ga in age, to test for evidence of a rise in atmospheric oxygen prior to the GOE or the so-called “whiff” event at 2.5 Ga. Individually, these isotope systems can provide useful information about the redox processes present in ancient sedimentary systems (Rouxel et al, 2005; Anbar and Rouxel, 2007; Johnson et al., 2008; Czaja et al., 2010; Heimann et al., 2010; Voegelin et al., 2010), but our data demonstrate that the combination of Fe and Mo isotopes provides powerful and quantitative evidence for free O2, including constraints on the minimum oxygen concentration required to produce co-variations in these isotopic systems.
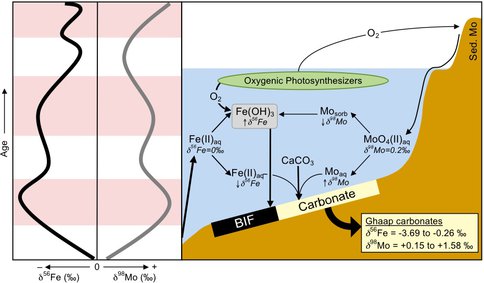
We measured the Fe isotope compositions of carbonate samples from drill core recovered from the Neoarchean–Paleoproterozoic Kaapvaal Craton of South Africa (Schröder et al., 2006; Knoll and Beukes, 2009), samples that were previously analyzed for Mo isotope compositions by Voegelin et al. (2010). Our analyses show that there is a generally negative correlation between δ56Fe and δ98Mo values (Figure 1; Czaja et al., submitted). This correlation is best explained by oxidation of reduced aqueous Fe [Fe(II)aq] by free O2 and the subsequent removal of Fe(III) oxyhydroxides [Fe(OH)3] from the photic zone. Figure 1 illustrates our model of coupled Fe and Mo isotope variation. Fe(II)aq with a δ56Fe value of ~0 ‰ was brought up from deeper in the basin, some of which was oxidized by photosynthetically produced oxygen to insoluble Fe oxy-hydroxides [Fe(OH)3] that had positive δ56Fe values. An oxygen flux to the atmosphere would have caused oxidative weathering of continental sulfides and the release of aqueous molybdate (MoO42-aq; δ98Mo = 0.2 ‰) to the ocean. During precipitation of Fe(OH)3, the light isotope of Mo is preferentially sorbed (Goldberg et al., 2009) and thus deposition of Fe(OH)3 as banded iron formation (BIF) or other Fe-rich sediments would have sequestered the positive δ56Fe and negative δ98Mo reservoirs. In turn, this would leave behind a residual pool of isotopically light Fe(II)aq and heavy MoO42- in the photic zone relative to their respective sources. We interpret such pools of residual dissolved Fe and Mo to have been directly incorporated into the carbonates sampled. Thus, the key control on the residual aqueous Fe and Mo isotope compositions are the relative proportions of initial aqueous Fe and Mo, and the amounts removed via Fe oxide formation and Mo sorption, respectively; this model provides the mechanism by which these two isotope systems may be coupled. Our model also suggests that times of increased Fe flux to marine sedimentary platforms and Fe deposition would have maximized the Fe and Mo isotope fractionations of the residual seawater. Consistent with this supposition, the periods of maximum negative Fe isotope and positive Mo isotope excursions¬ were all times of maximum flooding of the platform and Fe deposition (Figure 1), and this model is supported by analyses of the lithology and sedimentology of the platform (Beukes, 1987).
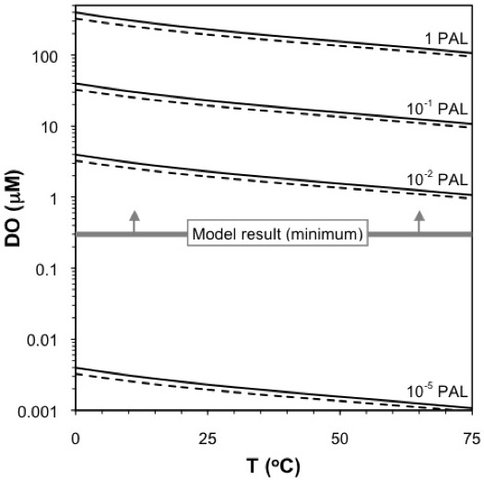
Based on our calculated initial Fe concentration of the water column, the stoichiometry of primary Fe oxides [Fe(OH)3], and the evidence for (at least) photic zone O2, we have estimated that the dissolved oxygen concentration for the water column in which these carbonates were deposited was 0.30 μM (Figure 2; Czaja et al., submitted). This value is ~10-3 times that expected for present-day seawater in equilibrium with the modern atmosphere (0.2 atm of O2). However, this value is at least 100 times higher than has been previously estimated immediately prior to the GOE at ~2.4 Ga (10-5 PAL; Holland, 2006 and references therein). Though our calculations only constrain the O2 content of the photic zone, at least some free atmospheric O2 would have been required to mobilize Mo during weathering, so it seems likely that both the atmosphere and oceans were significantly oxygenated at this time, although probably at a low level. The data reported here are also consistent with Fe and Mo isotope compositions measured from contemporaneously formed carbonates and shales from the Hamersley Province in Western Australia (Czaja et al., 2010), which suggests that this oxygenation was not a highly localized phenomenon. It is, however, important to recognize that it is likely these two continental blocks in the Archean (Cheney, 1996; Beukes and Gutzmer, 2008). Our results provide the first robust and quantitative estimate for O2 levels in the photic zone prior to the GOE, and further evidence that the atmospheric evolution of the Earth was complex in the Archean.
Figure 1. Schematic diagram of Ghaap Group carbonate Fe and Mo isotope compositions with time and a model of Fe and Mo cycling in the photic zone during deposition of the Neoarchean Campbellrand platform in South Africa. The left panel shows the coupled nature of Fe and Mo isotope compositions (black and gray curves, respectively). Pink bands mark times when a large amount of Fe was introduced to the platform relative to the times marked by white bands. The right panel illustrates our model of coupled Fe and Mo fractionation (see text for details). Fe oxy-hydroxide precipitation controls both the Fe and Mo isotope budgets for this shallow water system, making the δ56Fe value more negative and the δ98Mo value more positive with increasing Fe(OH)3 precipitation. The δ56Fe and δ98Mo values of the residual pool are recorded in the carbonates formed in this environment (box in the lower right). Adapted from Czaja et al., submitted.
Figure 2. Calculated values of dissolved oxygen content (DO) of seawater versus temperature (Chen, 1981). Calculations were based on various levels of atmospheric oxygen, expresed as proportions of the present atmospheric level of 0.21 atm (PAL) and salinity levels (thin solid lines = 20 ‰; thin dashed lines = 50 ‰). Photochemical models for mass-independent sulfure isotope fractionation suggest that prior to the Great Oxidation Event at ~2.4 Ga, the atmospheric O2 content was less than 10-5 PAL, and after this time it is inferred to have increased above 10-2 PAL, perhaps greater than 10-1 PAL (Holland, 2006). The thick gray line represents the the minimum DO value (0.30 µM) calculated in the present study (see text for explanation). Note the minor influence of temperature and salinity on dissolved oxygen contents compared to the orders of magnitude changes in the pO2 of the atmosphere over geologic time. Adapted from Czaja et al., submitted.
Publications
-
Czaja, A. D., Johnson, C. M., Beard, B. L., Eigenbrode, J. L., Freeman, K. H., & Yamaguchi, K. E. (2010). Iron and carbon isotope evidence for ecosystem and environmental diversity in the ∼2.7 to 2.5Ga Hamersley Province, Western Australia. Earth and Planetary Science Letters, 292(1-2), 170–180. doi:10.1016/j.epsl.2010.01.032
-
Czaja, A. D., Johnson, C. M., Roden, E. E., Beard, B. L., Voegelin, A. R., Nägler, T. F., … Wille, M. (2012). Evidence for free oxygen in the Neoarchean ocean based on coupled iron–molybdenum isotope fractionation. Geochimica et Cosmochimica Acta, 86, 118–137. doi:10.1016/j.gca.2012.03.007
-
Heimann, A., Johnson, C. M., Beard, B. L., Valley, J. W., Roden, E. E., Spicuzza, M. J., & Beukes, N. J. (2010). Fe, C, and O isotope compositions of banded iron formation carbonates demonstrate a major role for dissimilatory iron reduction in ~2.5Ga marine environments. Earth and Planetary Science Letters, 294(1-2), 8–18. doi:10.1016/j.epsl.2010.02.015
-
Johnson, C. M., Beard, B. L., Klein, C., Beukes, N. J., & Roden, E. E. (2008). Iron isotopes constrain biologic and abiologic processes in banded iron formation genesis. Geochimica et Cosmochimica Acta, 72(1), 151–169. doi:10.1016/j.gca.2007.10.013
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Brian Beard
Co-Investigator
Nicolas Beukes
Collaborator
Thomas Nägler
Collaborator
Andrew Czaja
Postdoc
Andrea Voegelin
Doctoral Student
-
RELATED OBJECTIVES:
Objective 4.1
Earth's early biosphere.
Objective 5.2
Co-evolution of microbial communities
Objective 6.1
Effects of environmental changes on microbial ecosystems
Objective 7.1
Biosignatures to be sought in Solar System materials