2010 Annual Science Report
 University of Wisconsin
Reporting | SEP 2009 – AUG 2010
University of Wisconsin
Reporting | SEP 2009 – AUG 2010
Project 2B: Production of Mixed Cation Carbonates in Abiologic and Biologic Systems
Project Summary
Carbonate minerals commonly occur on Earth and they are found in extraterrestrial materials such as meteorites and interplanetary dust particles. The chemistry of carbonates provides clues about their formation and alteration of over time. For example, carbonate minerals that form inorganically have chemical compositions that are highly constrained by the environmental conditions under which they grow, however, it is now known that microorganisms can produce carbonates that deviate from these generally accepted patterns. When carbonate minerals are placed in the appropriate environmental context, certain compositions may represent a biosignature for microbially mediated formation. The goal of this project is to develop a broader understanding of how carbonate minerals grow so that we may formulate explicit criteria for their origin based on their chemical and isotopic composition.
Project Progress
Year three accomplishments center primarily on the inorganic growth of Mg-bearing calcite from solution for the characterization of Mg-isotope fractionation relations in the calcite-aqueous Mg system. Pure calcite seed material was used as a substrate for the heterogeneous growth of Mg-calcite overgrowths from dilute solutions that contained dissolved MgCl2, CaCl2 and NaHCO3. Large volumes (50 L) of concentrated Ca, Mg and HCO3 stock solution (1 M) were made by dissolving the chloride and bicarbonate salts separately in CO2-equilibrated (1 atm) de-ionized (DI) water. Small aliquots of these solutions were mixed in varying proportion with CO2-equilibrated DI water to produce master solutions (500 mL) for individual experimental runs. The pH was controlled by bubbling a specified CO2-N2 gas mixture (350 ppm to 30% CO2) through each solution at a specified temperature (10°, 22° or 40°C). Re-equilibration of the solution at the reduced partial pressure of CO2 resulted in an increase in pH and the establishment of metastable supersaturated conditions with respect to Mg-calcite. Once pH was stabilized, pure calcite seed (~50 mg) was added to initiate the formation of Mg-calcite overgrowths. The pH was periodically monitored over the length of each experiment (1-3 days), and the duration of each experiment was selected to produce sufficient solid for Mg-isotope measurement while simultaneously maintaining solution chemistry within 10% of the initial solution composition. At the conclusion of each experiment, an aliquot of solution was filtered and immediately analyzed for Ca, Mg and alkalinity, and a second filtered sample was archived for Mg-isotope analysis. The remaining solid was separated by filtration, rinsed briefly in DI water and dried for further physicochemical characterization. Final solid weight was used to estimate the percentage of overgrowth for comparison to changes in solution chemistry. Aliquots of solid were characterized by x-ray diffraction and electron microprobe using EDS and WDS analytical techniques to determine the Mg-content of the overgrowth. The solid was also digested and analyzed for Ca and Mg content to confirm the mass and mole percent MgCO3 content of the overgrowth.
To date, eight experiments have been completed, with five yielding homogenous Mg overgrowths (2 to 9 mole% MgCO3) on calcite seed material (Fig. 1). In SEM images, Mg-overgrowths have rough crystal surfaces that display numerous growth steps and dislocations compared pure calcite overgrowths (Fig. 2).
In all of the experiments, the initial solution composition contained ~ 10 mmol Ca, ~45 mmol Mg and ~10 mmol HCO3. At 10°C, no Mg-overgrowth precipitated (Fig. 3), while at 22°C a ~2 mole% Mg overgrowth formed (Fig. 4), and at 40°C the same solution produced ~9 mole% MgCO3 overgrowth (Fig. 5). Multiple WDS spot analyses of the overgrowths demonstrate that Mg is distributed homogeneously throughout the overgrowth in each case. Maintaining a constant chemical composition for the overgrowth is important because preliminary Mg-isotope analyses suggest that isotope fractionation relations may be critically dependent on the chemical composition of the solid phase.
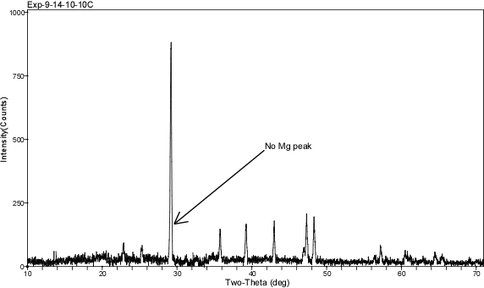
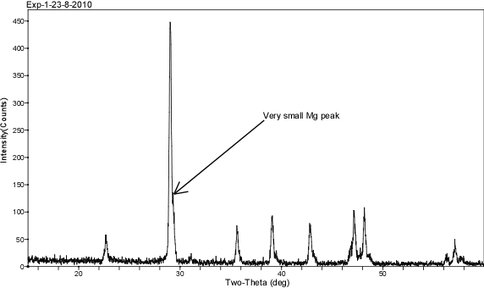
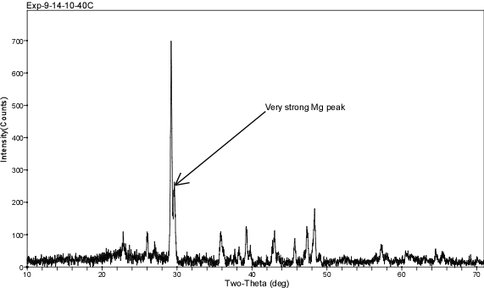
XRD diffractograms are displayed in Figs. 6 – 8 for these same experimental runs. The overgrowth is identified by a shoulder that develops on the main (104) calcite reflection at ~ 29° 2θ as the percentage of overgrowth increases. The difference in 2θ between the pure calcite and Mg-calcite (104) peak provides an estimation of the mol% MgCO3 in the overgrowth. These values are similar to those determined by WDS and EDs analysis and they are higher than measurements made by the bulk digestion of the solid phase.
Noteworthy results include:
1) Controlling the partial pressure of CO2 in solution is an effective means of controlling pH during an experimental run.
2) The use of a relatively large amount of seed material and short reaction time results in the generation of sufficient overgrowth for physicochemical characterization without having the solution chemistry change more than 10% over the course of a run.
3) Temperature is an effective means of controlling precipitation rate and the percentage of overgrowth in an experimental run.
4) The use of calcite seed materials promotes the formation of Mg-calcite overgrowth without cross contamination from other phases such as aragonite or hydromagnesite.
5) The WDS/EDS results suggest that chemically homogeneous overgrowths can be generated using the experimental system described above.
These results will form the basis for a broader suite of experiments to be completed to more fully characterize the effect of mol% MgCO3 on Mg isotope fractionation in the calcite-aqueous Mg system. Factors to be characterized include: 1) a wider range of mole% MgCO3 in the overgrowth, 2) the effect of PCO2, 3) the effect of precipitation rate, 4) the effect of temperature and 5) the effect of pH on Mg-isotope fractionation. If necessary, a more sophisticated experimental approach to precipitate a chemically homogeneous solid from a solution held under steady-state conditions (i.e., chemo-stat experiments) will be employed. In future years, the effect of additional cations (e.g., Fe, Mn) on Mg-isotope fractionation will be explored.
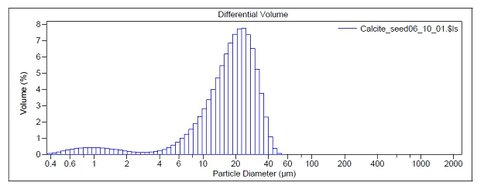
Figure 1.. Size distribution of calcite seed determined by laser diffraction particle size analysis.
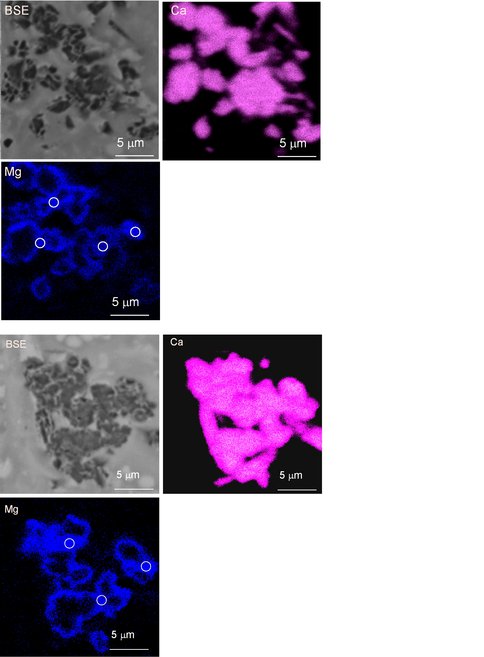
Figure 2. A).. Pure calcite over growth on calcite seed material. Fraction of overgrowth is ~70%.
Figure 2. B).. Homogeneous 5.7 mol% Mg-calcite over growth on calcite seed material. Fraction of overgrowth is ~70%.
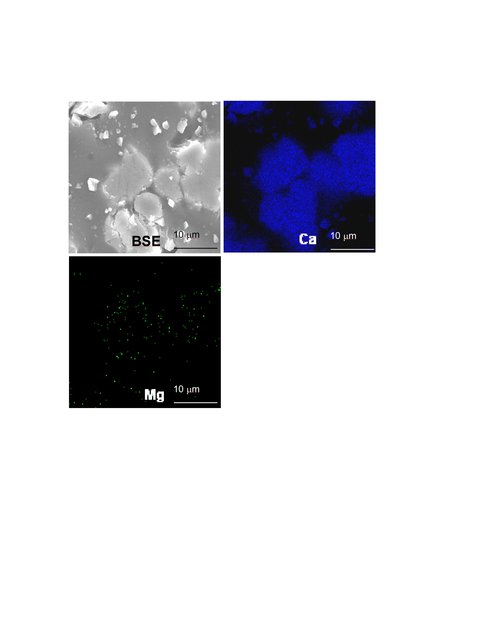
Figure 3.. Electron microprobe wave length dispersive spectroscopic (WDS) map of Ca (right panel) and Mg (left panel) from the final solid of run 9-14-10-10C at pH 7.27 and 10ºC. This experiment did not generate additional solid mass, suggesting that no overgrowth formed. The WDS map shows that recrystallization of the seed did not occur under these conditions.
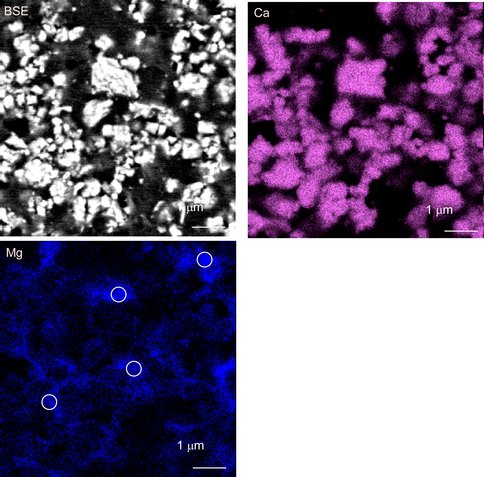
Figure 4.. Backscatter electron image and energy dispersive X-ray maps of final solid (20% overgrowth) produced during run 23-8-10 at 22ºC (pH = 7.19). White circles on the calcite grains represent the WDS analysis locations (Mg = 2.0 ± 0.4 mol% MgCO3 for n = 4).
Figure 5.. Backscatter electron image and energy dispersive X-ray maps of the final solid (47% overgrowth) produced during run 9-14-10-40C at 40ºC (pH = 7.42). White circles on the calcite grains represent the WDS analysis locations (Mg = 8.7 ± 0.80 mol% MgCO3 for n = 7).
Figure 6.. XRD diffractogram for solid from run 9-14-10-10C (pH = 7.27; 10ºC; see Fig. 3). The pattern shows no indication of a shoulder on the (104) calcite peak.
Figure 7.. XRD diffractogram for solid from run 23-8-10 (pH = 7.19; 22ºC; 20% overgrowth; 2.1 mol% MgCO3; see Fig. 4). The pattern shows a slight shoulder on the (104) calcite peak suggesting the presence of Mg-calcite overgrowth on the seed.
Figure 8.. XRD diffractogram for solid from run 9-14-10-40C (pH = 7.42; 40ºC; 47% overgrowth; 8.7 mol% MgCO3; see Fig. 5). The pattern shows a distinct shoulder on the (104) calcite peak which is diagnostic for the presence of a 9 mol% MgCO3 overgrowth.
Publications
-
Jimenez-Lopez, C., Romanek, C. S., & Bazylinski, D. A. (2010). Magnetite as a prokaryotic biomarker: A review. J. Geophys. Res., 115(G2), n/a–n/a. doi:10.1029/2009jg001152
-
Romanek, C. S., Morse, J. W., & Grossman, E. L. (2011). Aragonite Kinetics in Dilute Solutions. Aquat Geochem, 17(4-5), 339–356. doi:10.1007/s10498-011-9127-2
- Jimenez-Lopez, C., Rodriguez-Navarro, C., Perez Gonzalez, T., Rodriguez-Navarro, A., Bazylinski, D.A. & Romanek, C.S. (2010, In Review). Potential origin of Martian and Early Earth magnetites by thermal decomposition of (Ca,Mg,Fe)CO3. Nature-Geoscience.
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Max Coleman
Collaborator
Nadine Kabengi
Collaborator
Nita Sahai
Collaborator
Suvankar Chakraborty
Postdoc
-
RELATED OBJECTIVES:
Objective 4.1
Earth's early biosphere.
Objective 7.1
Biosignatures to be sought in Solar System materials