2010 Annual Science Report
 University of Wisconsin
Reporting | SEP 2009 – AUG 2010
University of Wisconsin
Reporting | SEP 2009 – AUG 2010
Project 2A: Chemolithotrophic Microbial Oxidation of Basalt Glass
Project Summary
Ferrous iron (Fe(II)) can serve as an energy source for a wide variety of chemolithotrophic microorganisms (organisms that gain energy from metabolism of inorganic compounds). Fe(II) oxidation may have played a role in past (and possibly, present) life on Mars, whose crust is rich in Fe(II)-bearing silicate minerals (e.g. ultramafic basalt rocks). The goal of this project is to determine whether an established chemolithoautotrophic Fe(II)-oxidizing, nitrate-reducing culture can grow by oxidation of Fe(II) in basalt glass. Experiments showed that the culture is able to oxidize a significant portion (approximately 1%) of the Fe(II) content of fresh basalt glass from Kilauea, a shield volcano in Hawaii that represents an analog for ancient volcanic activity on Mars. The ratio of Fe(II) oxidized to nitrate reduced was consistent with the expected 1:5 stoichiometry, suggesting that the culture oxidized Fe(II) with nitrate in a manner analogous to its metabolism of other (e.g. aqueous) Fe(II) forms.
Project Progress
Ferrous iron (Fe(II)) can serve as an electron donor for chemolithotrophic iron-oxidizing microorganisms under both oxic and anoxic conditions. It may also have played a role in past (and possibly, present) life on Mars, whose crust has a relatively rich content of Fe(II)-bearing silicate minerals (e.g. ultramafic basalt rocks) (McSween et al., 2009). Our initial goals were to determine whether an established chemolithoautotrophic Fe(II)-oxidizing, nitrate-reducing culture (Blothe & Roden, 2009 ; Straub et al., 1996 ; Weber et al., 2001) can grow by oxidation of Fe(II) in basalt glass. Previous and ongoing NAI-supported studies have demonstrated that this culture is capable of growing with insoluble Fe(II)-bearing phyllosilicate phases such as biotite, smectite, and illite. Our interest in the potential for microbial oxidation of Fe(II) in basalt glass stems from recent suggestions that near-surface hydrothermal venting may have occurred during past periods of active volcanic/tectonic activity on Mars (Neukum et al., 2010). Such activities could have produced basalt glass phases that might have served as energy sources for chemolithotrophic microbial activity.
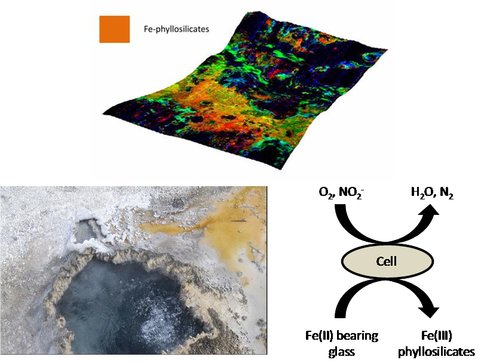
Figure 1. Magma from erupting volcano rapidly cools to form glass (A). Glass settles on shore of hot spring (A) where it is consumed by Fe(II) oxidizing bacteria©. Possible end products may include Fe(III) bearing phyllosilicates, such as those indicated by a mineral map of CRISM image HRL0000285A.
Several experiments were conducted to determine whether the Fe(II)-oxidizing, nitrate-reducing culture can oxidize Fe(II) in basaltic glass. Previous and ongoing NAI-supported studies have demonstrated that this culture is capable of growing with insoluble Fe(II)-bearing phyllosilicate phases such as biotite, smectite, and illite. Our interest in the potential for microbial oxidation of Fe(II) in basalt glass stems from recent suggestions that near-surface hydrothermal venting may have occurred during past periods of active volcanic/tectonic activity on Mars. Such activities could have produced basalt glass phases that might have served as energy sources for chemolithotrophic microbial activity. Thus we sought to test whether the culture could oxidize the Fe(II) in basalt glass in a manner analogous to its ability to oxidize Fe(II) in insoluble Fe(II)-bearing phyllosilicate phases. The glass was obtained from an active lava flow from the Kilauea volcano in Hawaii.
Figure 2. USGS geologist sampling a lava tube (A). Rapid quenching of the lava in water produces chunks of basalt glass (B, upper), which were powderized and separated into clay sized materials (B, lower) for use as an energy source for the Fe(II)-oxidizing, nitrate-reducing culture©.
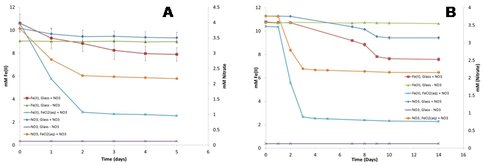
As a shield volcano, Kilauea has long stood as a Mars analog. The culture was initially grown on clay-sized particles of crushed glass suspended in anaerobic liquid media with nitrate as the electron acceptor. The results indicate chemolithotrophic Fe(II) oxidation took place: the dilute HCl-extractable Fe(II) content of the mineral suspensions declined
Figure 3. (A) Fe(II) oxidation and nitrate consumption in a culture inoculated with aqueous Fe(II)-grown cells (Generation 1). (B) Fe(II) oxidation and nitrate consumption in a culture inoculated with cells from Generation 1. Data points show the mean ± SD quadruplet cultures.
over time in inoculated suspensions, whereas no significant Fe(II) loss took place in uninoculated controls. Near-term future work will involve X-ray diffraction and electron microscopic analysis of the Fe phases produced during microbial Fe(II) oxidation. Parallel analyses will be conducted on glass samples oxidized abiotically by H2O2 to determine whether enzymatic activity produces compositionally or morphologically unique end-products.
References Cited
Blothe M, Roden EE (2009) Composition and activity of an autotrophic Fe(II)-oxidizing, nitrate-reducing enrichment culture. Applied and Environmental Microbiology, 75, 6937–6940.
McSween HY, Taylor GJ, Wyatt MB (2009) Elemental composition of the Martian crust. Science, 324, 736-739.
Neukum G, Basilevsky AT, Kneissl T, Chapmam MG, van Gasselt S, Michael G, Jaumann R, Hoffmann H, Lanz JK (2010) The geologic evolution of Mars: Episodicity of resurfacing events and ages from cratering analysis of image data and correlation with radiometric ages of Martian meteorites. Earth Planet. Sci. Lett., 294, 204-222.
Straub KL, Benz M, Schink B, Widdel F (1996) Anaerobic, nitrate-dependent microbial oxidation of ferrous iron. Applied and Environmental Microbiology, 62, 1458-1460.
Weber KA, Picardal FW, Roden EE (2001) Microbially-catalyzed nitrate-dependent oxidation of biogenic solid-phase Fe(II) compounds. Environmental Science & Technology, 35, 1644-1650.
Publications
- Hong, K-S. (2010). Piezoelectrochemcial Effect: Mechanical Energy Induced Redox Reaction in Aqueous Solutions through Vibrating Piezoelectric Materials. University of Wisconsin-Madison.
- Honma, A. & Roden, E.E. (2010). Chemolithotrophic microbial oxidation of basalt glass. Astrobiology Science Conference 2010, Abstract 5367.
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Evgenya Shelobolina
Research Staff
Andrew Honma
Graduate Student
-
RELATED OBJECTIVES:
Objective 2.1
Mars exploration.
Objective 6.2
Adaptation and evolution of life beyond Earth
Objective 7.1
Biosignatures to be sought in Solar System materials