2010 Annual Science Report
 University of Wisconsin
Reporting | SEP 2009 – AUG 2010
University of Wisconsin
Reporting | SEP 2009 – AUG 2010
Project 1B: Proto-Cell Membrane Evolution May Have Been Directed by Mineral Surface Properties
Project Summary
In the present project, we used both experimental and classical modeling approaches to examine the novel hypothesis that the surface chemistry of minerals in contact with self-assembling lipid vesicles (i.e., model protocells) controlled the stability of the vesicle membrane, causing membranolysis and preventing self-assembly in the case of minerals with certain surface properties, while other minerals were relatively inert. We also examined the role of solution chemistry and temperature cycling on lipid vesicle stability in contact with mineral surfaces. By understanding the role of natural geochemical parameters such as mineral surface chemistry, solution chemistry (pH, ionic strength, presence or absence of Ca2+), and temperature cycling on protocell membrane stability under variable conditions, we attempted to model potential aqueous environments where life may have originated such as lacustrine, tidal pool, and sub-aerial or submarine hydrothermal vents. Our project addresses NASA Astrobiology Institute’s (NAI) Roadmap goals of understanding the origins of cellularity and the evolution of mechanisms for survival at environmental limits, and NASA’s Strategic Goal of advancing scientific knowledge of the origin and evolution of the Earth’s biosphere and the potential for life elsewhere.
Project Progress
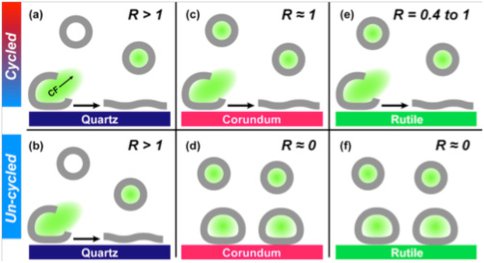
We used phospholipid vesicles of various charges, zwitterionic dipalmitoylphosphatidylcholine (DPPC), anionic dipalmitoylphosphatidylserine (DPPS), and cationic dipalmitoylethylphosphatidylcholine(DPEPC), in order to represent prebiotic protocells although we recognize that phospholipids are biologically produced, and that simpler lipids would have existed pre-biotically. We used quartz (α-SiO2), corundum (α-Al2O3) and rutile (α-TiO2) particles (~ 1 μm diameter) as model minerals, in order to represent a range of surface charges, dielectric constants, and mineral solvation properties, and because the surface chemistry of these oxides is well-established in the literature. Using fluorimetric experiments on flurorescent dye-loaded DPPC vesicles in the presence of mineral particles, we determined that the greatest extent of DPPC vesicles was induced by quartz surfaces, followed by corundum and the least rupture was obtained on rutile (Figure 1). Furthermore, vesicle rupture occurred on quartz regardless of thermal cycling across the lipid gel–liquid crystal phase transition temperature, but rupture was promoted by cycling on corundum and rutile. If we consider vesicle rupture as a model process for membranolysis of protocells, our results suggest that quartz has a greater ability to induce membranolysis than the other two mineral surfaces. After rupturing in contact with all three mineral surfaces, the lipid molecules self-assembled at the surfaces into bilayers similar to model membranes (Figure 1). Adsorption isotherms of DPPC at pH 7.2 in 0.017 M NaCl solution at 55°C (above DPPC transition temperature) showed only two lipid bilayers on quartz compared to three on corundum and rutile (Figure 2). These results, again, indicated a lower affinity of the DPPC bilayers on the quartz surface, which is negatively-charged and slightly less hydrophilic than the other two mineral surfaces. The changes in the number of bilayers obtained at various pHs, and at various ionic strengths set by NaCl, with or without Ca2+, were explained by our modified Deraguin-Landau-Verwey-Ovebeek (DLVO) theory for colloidal stability (Figures 2 and 3). If DPPC bilayers were assumed to bear a small negative potential, then van der Waals forces predominantly accounted for two DPPC bilayers, and adsorption beyond the second bilayer occurred at low ionic strength due to extension of the electric double-layer near the oxide surface. Inclusion of bilayer–bilayer interactions resulted in a prediction of infinite DPPC bilayer deposition irrespective of the oxide. This was not observed experimentally, perhaps, due to the systems reaching quasi steady-state conditions rather than equilibrium. In contrast, adsorption isotherms of anionic DPPS and cationic DPEPC lipids show that adsorption of highly charged bilayers was decreased or prevented altogether due to bilayer-oxide and/or bilayer–bilayer repulsion (Figure 2).
In summary, mineral surface potential and relative hydrophilicity, lipid bilayer surface potential, and solution composition and temperature cycling, among other factors, could have controlled the self-assembly or lysis of early proto-cell membranes composed of amphiphilic lipids or lipid precursors such as fatty acids and fatty acid esters. Changes in solution conditions such as pH or ionic strength due to wetting–drying cycles in a tidal or lacustrine environment or due to a chemical gradient in seawater, for e.g., with distance from a submarine hydrothermal vent, could lead to different numbers of bilayers associated with mineral surfaces. Our results suggest that the self-assembly and stability of early proto-cell lipid membranes would have been favored on surfaces of minerals with positively-charged and more hydrophilic nature, and which were more abundant than the model corundum used here, compared to minerals with negatively-charged and less hydrophilic surfaces such as quartz or minerals of similar surface chemistry.
Figure 1. Schematic summary of fluorescence experiments showing extent of vesicle rupture (Rupture Factor R) for fluorescent-dye loaded DPPC vesicles interacting with quartz, corundum, and rutile particle suspensions at pH 7.2 and ionic strength 17 mM NaCl at 25oC, when cycled or not cycled through the lipid phase-transition temperature. Green-filled vesicles indicate that CF cargo is still present, while empty vesicles denote rupture of additional, non-adsorbed vesicles. R ≥ 1 implies 100% vesicle rupture on contact with mineral surface, and R < 1 implies fractional vesicle rupture. Illustration is not to scale (Oleson et al., 2010).
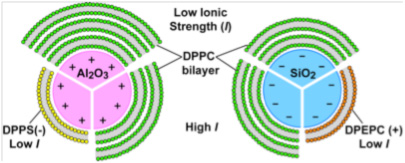
Figure 2. Conceptual illustration of changes in mutiple lipid bilayer deposition at corundum and quartz surfaces obtained by measuring adsorption isotherms. The variations in the number of deposited lipid bilayers can be explained on the basis of extended DLVO theory, modified to account for solvent exclusion from the interfacial region by the hydrophobic lipid tails, thus resulting in spatial extension of the electric double layer. Legend: Green, yellow and orange small spheres – DPPC, DPPS and DPEPC lipid head groups, grey shaded areas – tail regions of phospholipid bilyers, white areas – intervening aqueous solution between bilayers, pink sphere with positive surface charge – corundum particles, and blue sphere with negative surface charge – quartz particles (Oleson et al., 2010).
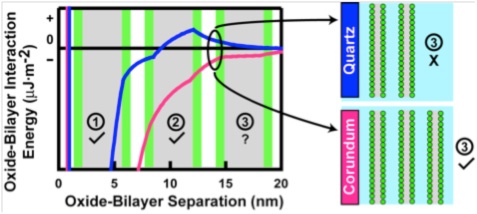
Figure 3. Conceptual illustration of oxide-bilayer interaction energies calculated according to extended DLVO theory, modified to account for solution exclusion by bilayers, helps to explain experimentally-observed differences in number of bilayers deposited on corundum versus quartz (Oleson and Sahai, 2010). Legend: as in Figure 2.
Publications
-
Oleson, T. A., & Sahai, N. (2010). Interaction energies between oxide surfaces and multiple phosphatidylcholine bilayers from extended-DLVO theory. Journal of Colloid and Interface Science, 352(2), 316–326. doi:10.1016/j.jcis.2010.08.056
-
Oleson, T. A., Sahai, N., & Pedersen, J. A. (2010). Electrostatic effects on deposition of multiple phospholipid bilayers at oxide surfaces. Journal of Colloid and Interface Science, 352(2), 327–336. doi:10.1016/j.jcis.2010.08.057
-
Xu, J., Stevens, M. J., Oleson, T. A., Last, J. A., & Sahai, N. (2009). Role of Oxide Surface Chemistry and Phospholipid Phase on Adsorption and Self-Assembly: Isotherms and Atomic Force Microscopy. The Journal of Physical Chemistry C, 113(6), 2187–2196. doi:10.1021/jp807680d
- Oleson, T.A. & Sahai, N. (2008). Oxide-dependent adsorption and self-assembly of dipalymitoylphosphatidylcholine, a cell-membrane phospholipid: Bulk Adsorption Isotherms. Langmuir, 24: 4865-4873.
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Timothy Oleson
Doctoral Student
Jie Xu
Doctoral Student
-
RELATED OBJECTIVES:
Objective 3.4
Origins of cellularity and protobiological systems
Objective 5.1
Environment-dependent, molecular evolution in microorganisms