2010 Annual Science Report
 Rensselaer Polytechnic Institute
Reporting | SEP 2009 – AUG 2010
Rensselaer Polytechnic Institute
Reporting | SEP 2009 – AUG 2010
Project 7: Prebiotic Chemical Catalysis on Early Earth and Mars
Project Summary
The “RNA World” hypothesis is the current paradigm for the origins of terrestrial life. Our research is aimed at testing a key component of this paradigm: the efficiency with which RNA molecules form and grow under realistic conditions. We are studying abiotic production and polymerization of RNA by catalysis on montmorillonite clays. The catalytic efficiency of different montmorillonites are determined and compared, with the goal of determining which properties distinguish good catalysts from poor catalysts. We are also investigating the origin of montmorillonites, to test their probable availability on the early Earth and Mars, and the nature of catalytic activity that could have led to chiral selectivity on Earth.
Project Progress
This project aims to test the “RNA World” hypothesis for the origin of life. We are investigating the nature and origin of catalytic clays, the catalytic process that leads to RNA production, and the possible role of catalysis in the origin of homochirality. Progress on each sub-project is described below.
The nature and origin of catalytic clays
Chemical analyzes and x-ray diffraction studies have been acquired on ~15 montmorillonite-rich bentonites previously assessed for their ability to catalyze RNA oligomerization. Chemical and crystallographic features have been identified that occur systematically within the catalytically active samples, but are absent from the inactive samples. These features and the specific geological settings that generated these samples have led to a hypothesis that is undergoing further tests, and will soon be submitted for journal review. An abstract and poster on this work were presented by Delano at the 41st Lunar and Planetary Science Conference (LPSC 2010).
Homochirality in the RNA World
Studies from our laboratory demonstrated the montmorillonite clay-catalyzed formation of RNA oligomers from activated monomers (Ferris 2002; see Figures 1 and 2). The longest oligomers determined by HPLC analysis were seven mers from D, L-ImpA and six mers from D, L-ImpU (Joshi et al. 2007). The low homochirality and the short oligomers indicate that this is not an encouraging approach to the formation of RNA oligomers that have catalytic activity.
The different approach to the formation of the RNA was the reaction of a quaternary mixture of D, L-ImpA with D, L-ImpU. This approach was chosen because D, L- mixtures would have been more likely to have formed spontaneously on the prebiotic Earth. When a mixture of D, L-ImpA reacted with D, L-ImpU we obtained oligomers as long as 11-mers using a HPLC ion exchange column. The products were identified by RNase T2 hydrolysis which cleaves D-3’, 5’ links but not the L-links because the latter are only compatible with D-compounds. The percent of homochirality of the products was measured for the three independent reactions (Table 1; Joshi et al. 2009). Of particular interest was the increase of homochirality from 64% to 76% while D, L-ImpA and D, L -ImpU had major decreases from 67% to 33% and 39% to 11%, respectively (Joshi et al. 2010). Further studies are in progress on the identification of the longer oligomers and the determination of their homochirality.
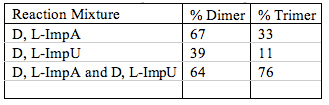
Table 1. Homochirality of the reaction products.
In addition to investigation of the organic products formed by the montmorillonite catalysis of activated RNA monomers we are also investigating the mechanism of the catalysis. The extent of catalysis depends on the magnitude of the negative charge on the montmorillonite and the negative charge on the activated monomers. The optimal binding of the activated monomers to the montmorillonite occurs in the region of maximal oligomer formation. X-ray diffraction studies revealed changes in layer separations in the montmorillonite as the reaction occurs. The application of the Scherrer equation to the X-ray diffraction data showed differences in domain size. Modeling of the size of the activated nucleotide monomers and the charge on the montmorillonite surface provided an interpretation of how these factors influence adsorption. These findings provide a basis for further understanding of the physical processes in the mechanism of this catalysis.
Enhanced Detection of Abiotically Synthesized RNA in MALDI-MS
McGown’s work focuses on the abiotic synthesis of RNA on montmorillonite clay surfaces. Specifically, we have been studying polymerization of activated ribonucleotides ImpA and ImpU alone and in mixtures. A key aspect of this work is the detection of the polymerization products using Matrix-Assisted Laser Desorption Ionization-Mass Spectrometry (MALDI-MS). In the course of our experiments, we discovered that MALDI-MS detection of RNA oligonucleotides could be extended to higher mass ranges by using fused silica instead of metal targets. Metal is the standard desorption platform for MALDI-MS and it is generally believed that the use of fused silica reduces detectability especially at higher mass ranges. We were therefore surprised by the enhancement that we observed for RNA oligomers.
ImpA, ImpU and ImpA products extracted from the montmorillonite clay were analyzed on stainless steel and fused silica targets. Conditions were individually optimized for the samples on each of the surfaces. The results showed enhanced detection for all three polymers. For polyA, detection extended only as far as the 12-mer on steel but could be extended to the 18-mer on silica. For polyU, detection was extended from 11-mer on steel to the 14-mer on silica. For polyA/U, detection was extended from the 10-mer on steel to the 12-mer on silica. In all cases, signals were greatly enhanced on silica compared to steel for the 7-mers and beyond. Although MALDI-MS generally is not a quantitative tool, the actual intensities of the various peaks likely reflect the actual abundances of those species in the extracted products, combined with their MALDI efficiencies. These results are highly relevant to rapid screening of abiotic RNA polymerization toward elucidating pathways to life on Earth.
Collecting samples of montmorillonite clays from the bentonite layer at a field site near Morden, Manitoba (October 2009).
References
Ferris, J. P., 2002, “Montmorillonite Catalysis of 30-50 mer Oligonucleotides: Laboratory Demonstration of Potential Steps in the Origin of the RNA World”, Origins Life Evol. Biospheres, 32, 311
Joshi, P. C., Pitsch, S., & Ferris, J. P. 2007, “Selectivity of Montmorillonite Catalyzed Prebiotic Reactions of D, L-Nucleotides”, Origins Life Evol. Biospheres, 37, 3
Joshi, P. C., Aldersley, M. F., Delano, J. W., & Ferris, J. P. 2009, “Mechanisms of Montmorillonite Catalysis in the Formation of RNA Oligomers”, J. Am. Chem. Soc., 131, 13369
Joshi, P. C., Aldersley, M. F., & Ferris, J. P. 2010, “Homochiral Selectivity in RNA Synthesis: Montmorillonite-catalyzed Quaternary Reactions of D, L-Purine and D, L-Pyrimidine Nucleotides”, Origins Life Evol. Biospheres, in press
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Michael Aldersley
Research Staff
Prakash Joshi
Research Staff
Lauren Cassidy
Graduate Student
John Grossman
Undergraduate Student
Alex Meola
Undergraduate Student
Matthew Moellman
Undergraduate Student
Joseph Thompson
Undergraduate Student
-
RELATED OBJECTIVES:
Objective 3.1
Sources of prebiotic materials and catalysts
Objective 3.2
Origins and evolution of functional biomolecules

