2010 Annual Science Report
 Rensselaer Polytechnic Institute
Reporting | SEP 2009 – AUG 2010
Rensselaer Polytechnic Institute
Reporting | SEP 2009 – AUG 2010
Project 6: The Environment of the Early Earth
Project Summary
This project involves the development of capabilities that will allow scientists to obtain information about the conditions on early Earth (3.0 to 4.5 billion years ago) by performing chemical analyzes of crystals (minerals) that have survived since that time. When they grow, minerals incorporate trace concentrations of ions and gaseous molecules from the local environment. We are conducting experiments to calibrate the uptake of these “impurities” that we expect to serve as indicators of temperature, moisture, oxidation state and atmosphere composition. To date, our focus has been mainly on zircon (ZrSiO4), but we have recently turned our attention to quartz as well.
Project Progress
The last 12 months have seen steady progress towards the completion of, or significant contribution to, several of our main research themes. A significant body of research, incorporating >120 individual experiments run over a total of ~5000 hours, has been carried out under three separate projects at Rensselaer (New York) Astrobiology Center. These individual projects, while diverse, all fall under themes outlined in section 7.2of our 2008 NAI proposal (uptake of 'indicator species’ in ancient zircons and other silicates).
Ce anomalies in zircon (ZrSiO4): indicators of oxygen fugacity and melt composition. The unique properties of zircon,
The unique properties of zircon make the mineral among the most important and extensively studied from the Hadean and early Archean (4.5-3.0 Ga). These properties range from (but are not limited to) hardness and durability (i.e., the ability to withstand both chemical and physical attack) to the incorporation of a number of trace elements. The incorporation of radioactive elements Uranium and Thorium into zircon, for example, make it possible to date zircon crystallization. Another group of elements that are known to substitute into zircon are the “Rare Earth Elements” (otherwise known as elements of the Lanthanide series). The elements from lanthanum through lutetium generally behave in a similar pattern because they all have similar size (ionic radii), and they are all generally found in the trivalent state (e.g., La3+). There are two exceptions to these conditions:
1) Cerium can be found in the trivalent (Ce3+ ) and tetravalent (Ce4+) state, and
2) Europium can be found in the trivalent (Eu3+) or divalent (Eu2+) state.
The polyvalent behavior of these two elements often results in disruptions from an otherwise smooth chemical trend from lanthanum to lutetium in minerals. This is to say the Cerium is often enriched and Europium depleted relative to the other lanthanides.
Enrichments in cerium (positive anomaly) are generally attributed to crystallization within an oxidizing environment while depletions in europium (negative anomaly) are generally attributed to crystallization within more reducing environments. In order to gain some insight into the potential use of polyvalent behavior among these two lanthanides, and in order to develop a new environmental indicator (applicable to sample of the Hadean and early Archean), experiments were carried out at different oxygen fugacities and melt compositions. In other words, we are trying to get a gauge on the environmental conditions responsible for these anomalous excursions (positive and negative) within the Lanthanide series.
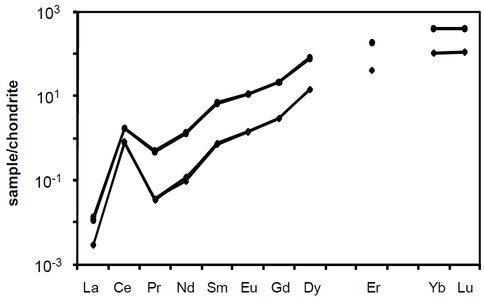
Figure 1. Ten rare earth element (REE) profiles from natural zircons showing positive Ce anomalies (after Tailby et al., in press). Note that with increasing atomic number there is a smooth increase in elemental concentration (corresponding to decreasing ionic radii), with the exception of Ce. This positive Ce anomaly is thought to be sensitive, and reflets the existence of tetravalent (Ce4+) and trivalent (Ce3+) states.
Results from this study demonstrate that a systematic increase in zircon Ce anomalies correlate with higher oxygen fugacities and lower crystallization temperatures. Similarly, Eu anomalies become more negative at lower oxygen fugacities. These observations confirm the hypothesis that polyvalent behavior among the Lanthanides, at least in part, reflects changes in oxygen fugacity. Another interesting result observed from this work is that the magnitude of the positive (Ce) or negative (Eu) anomaly is also largely dependent on melt. This suggests that polyvalent behavior can be used not only as an oxygen sensor, but also as a probe into the type of melt that ancient zircons crystallized.
Trace element incorporation into quartz.
The second avenue of investigation involves quartz (SiO2), a mineral common to the continental crust and found within a broad range of geological environments. Like zircon, quartz is a mineral displaying a number of properties (e.g., hardness) that can result in the mineral persisting over very long geological timescales. Many ancient sedimentary rocks, like the Witswatersrand conglomerate, show quartz as the dominant rock-forming mineral. This relatively simple mineral is also characterized by compositions close to purity, with very few elements known to substitute within the crystal lattice. The select group of elements that are known to substitute within quartz (generally at concentrations <100-10 ppm) include Al, Ti, Fe, P, Li, K, Na and H+.
It has recently been demonstrated that the Ti content of quartz can be used to estimate the temperature of crystallization, assuming growth under specific conditions. This titanium in quartz example has demonstrated that potential exists for quartz as a geological indicator. The development of additional environmental indicators in quartz is highly advantageous because the mineral is well documented and abundant in a range of ancient rocks.
In this experimental study we have included a broader range of elements (including Al, Fe, P and H) in an effort to better understand how quartz composition changes as a function of temperature, pressure, oxygen fugacity and system composition. In other words, this experimental research is aimed at increasing the potential use of natural quartz as a geological indicator.
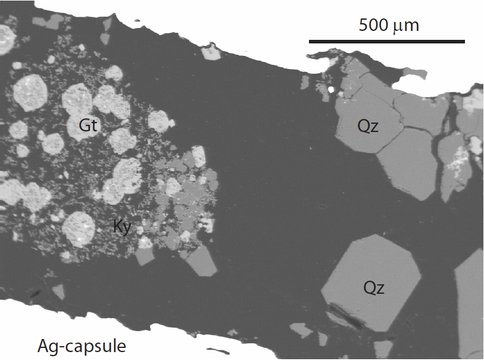
Figure 2. A typical quartz experiment from our research. This photomicrograph shows grains of garnet (Fe-rich phase; Gt), kyanite (and aluminous phase; Ky) and quartz (Qz). This experiment, run at 2 GPa/750 ºC is aimed at determining Al- and Fe-substitution in quartz.
Our results vary depending on pressure-temperature-bulk composition, but a number of systematic trends are present. The aluminum concentration of quartz, generally the most abundant trace element within the mineral, is particularly promising. The aluminum concentration of quartz decreases notably as a function of temperature. Similarly, increases in pressure are accompanied by decreases in aluminum concentration in quartz. These combined observations demonstrate how the Al- and Ti-concentration of quartz may be used as a geological indicator (or thermobarometer).
Diffusion of divalent cations in carbonates.
Carbonate minerals are common on planetary bodies, and on Earth itself they are generally biogenic in origin. In principle, a record of formation conditions (and possible biogenic origin) is contained within the carbonate crystal structure in the concentrations and isotopic compositions of C, O, Fe, Mn, Ca and Mg. Knowledge of the diffusion behavior of these components is therefore essential to understanding the reliability of these key indicators of carbonate origin. It is within this context that we are examining interdiffusion phenomena involving Fe, Mn, Ca and Mg in carbonates. The diffusion properties of these elements are not well constrained at low temperature, and robust diffusion laws are needed in order to extrapolate with confidence to conditions of shallow burial in the Earth and Mars [see Michalski et al. (2010) Nature Geoscience. doi:10.1038/ngeo971]. Our experiments investigate coupled diffusive element exchange involving Fe, Mn and Ca between co-existing carbonate phases in the range of 400-625 °C at 1atm. Results and subsequent modeling suggest that natural carbonate grains are likely to preserve information not only on the conditions of formation but also any subsequent alteration during metamorphic events at low to medium temperatures on timescales typical for contact or regional metamorphism. Currently, experiments and analytical work are nearly complete and a manuscript is undergoing internal review.
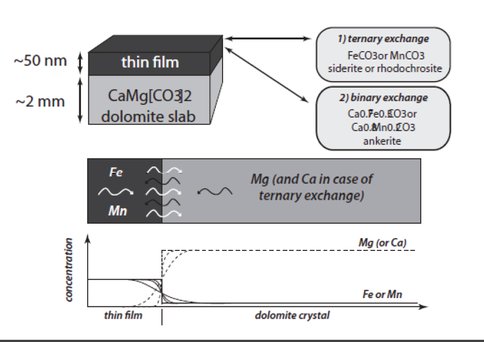
Figure 3. Schematic illustration of the experimental setup. Diffusion couples were produced by depositing thin films of known composition onto cleaved and cleaned surfaces of pure dolomite crystals. During the diffusion anneals in a 1-atm furnace at elevated temperatures in a stream of CO2 gas, binary or multicomponent exchange will occur by coupled diffusion depending on the composition of the thin film. Following the experiments, samples were analyzed using Rutherford Backscattering Spectroscopy (RBS) and concentration profiles in the near-surface region were extracted to determine the diffusion coefficients (see text for details).
Biosignature mimics – mass dependent carbon isotope fractionation.
The abundances and isotopic compositions of C, S, N, H as well as redox-sensitive elements such as Fe, Mn or Mo are used as so-called ‘biosignatures’ in Astrobiology. The basic idea behind these indicators is to relate specific compositions measured in rocks to ancient environmental conditions or processes in order to find evidence for the onset of life on terrestrial or planetary bodies. For example, biological activity is known to modify (specifically, to make lighter) the carbon isotopic ratio (δ13C), so it is tempting to attribute light carbon signatures in minerals of ancient rocks to the metabolic activity of living organisms. Caution is nevertheless required in this endeavor, because it has been shown recently that inorganic processes such as diffusive transport of atoms through minerals and rocks by diffusion can also fractionate isotope ratios. Indeed, stable isotopes of carbon show a range of ~8% in rocks- and it is imperative to determine what extent of this range is attributable unequivocally to life. To this end, we undertook an experimental study of carbon diffusion in the grain boundaries of rocks and in Fe metal, with a view toward determining the effect of isotopic mass on diffusion. Our intent is to explore the potential of high-temperature diffusion to produce a “life-like” fractionation of carbon isotopes. At this juncture we have completed an initial series of experiments and have reserved ion microprobe time to perform the analyses in the weeks ahead.
Publications
-
Müller, T., Cherniak, D., & Bruce Watson, E. (2012). Interdiffusion of divalent cations in carbonates: Experimental measurements and implications for timescales of equilibration and retention of compositional signatures. Geochimica et Cosmochimica Acta, 84, 90–103. doi:10.1016/j.gca.2012.01.011
-
Tailby, N. D., Walker, A. M., Berry, A. J., Hermann, J., Evans, K. A., Mavrogenes, J. A., … Sutton, S. R. (2011). Ti site occupancy in zircon. Geochimica et Cosmochimica Acta, 75(3), 905–921. doi:10.1016/j.gca.2010.11.004
-
Trail, D., Bruce Watson, E., & Tailby, N. D. (2012). Ce and Eu anomalies in zircon as proxies for the oxidation state of magmas. Geochimica et Cosmochimica Acta, 97, 70–87. doi:10.1016/j.gca.2012.08.032
-
Trail, D., Thomas, J. B., & Watson, E. B. (2010). The incorporation of hydroxyl into zircon. American Mineralogist, 96(1), 60–67. doi:10.2138/am.2011.3506
- Mojzsis, S.J., Cates, N.L., Maier, A.C., Abramov, O., Trail, D., Bleeker, W. & Guitreau, M. (2010). To see the Headean in a slab of gneiss. AGU 2010. San Francisco, California, USA.
- Müller, T., Cherniak, D. & Watson, E.B. (2010). Application of Diffusion Data in Carbonates to Estimate Timescales and Conditions of Texture Forming Processes. EOS Trans. AGU, 90.
- Tailby, N., Thomas, J.B. & Watson, E.G. (2010). Trace elements in quartz: experimental constraints on Al, Ti, Fe and P saturation. Geochim. Cosmochim. Acta, 74(12): A1020.
- Trail, D., Thomas, J.B. & Watson, E.B. (2009). OH in zircon. Geochim. Cosmochim. Acta, 73(13): A1343.
- Trail, D., Watson, E.B. & Tailby, N. (2010). Melt composition and oxygen fugacity influence on Ce and Eu anomalies in zircon. GSA 2010. Denver, Colorado USA.
- Trail, D., Watson, E.B. & Tailby, N. (2010). New experimental constraints for Hadean zircon source melts from Ce and Eu anomalies in zircon. AGU 2010. San Francisco, California, USA.
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Suzanne Baldwin
Co-Investigator
John Delano
Co-Investigator
Thomas Mueller
Postdoc
Nick Tailby
Postdoc
Dustin Trail
Graduate Student
Sebastian Mergelsberg
Undergraduate Student
Egidio Tentori
Undergraduate Student
-
RELATED OBJECTIVES:
Objective 1.1
Formation and evolution of habitable planets.
Objective 4.1
Earth's early biosphere.
Objective 4.3
Effects of extraterrestrial events upon the biosphere