2010 Annual Science Report
 Montana State University
Reporting | SEP 2009 – AUG 2010
Montana State University
Reporting | SEP 2009 – AUG 2010
X-Ray Characterization of Modified Fe-S Mineral Surfaces
Project Summary
High energy X-ray radiations generated by tunable synchrotron lightsources were used to characterize both the location of the electrons and the atomic centers in modified Fe-S minerals and particles. We have exploited the complementary information content of three different detections techniques in both soft and hard X-ray energy range. We confirmed the formation of a reduced pyrite structure from hydrogen atom exposure experiments. The formation of a reduced pyrite surface is relevant to small molecule activation processes of abiotic molecules toward formation of more complex molecules, such as amino and nucleic acids.
Project Progress
In addition to the FTIR spectroscopic and SEM imaging techniques we have extended the characterization techniques of surface species to multi-edge X-ray spectroscopy. This emerging spectroscopic technique uses high intensity, tunable X-rays from both the traditional soft (<1 keV) and hard (> a few keV) X-rays, as well as the intermediate ‘tender’ X-ray energy range to obtain complementary structural information about the structure of a given absorber (Fe or S). Furthermore, we exploited the benefits of using various detection techniques that allowed us to gain insights into the depth profile of the molecular beam exposed pyrite samples. After exposure to D/Ar beam, the pyrite samples were removed from the vacuum chambers and exposed to air. The unavoidable oxidation of the top surface turned out to be beneficial due to formation of a protective cap for preserving the catalytically active partially reduced Fe-S layer.
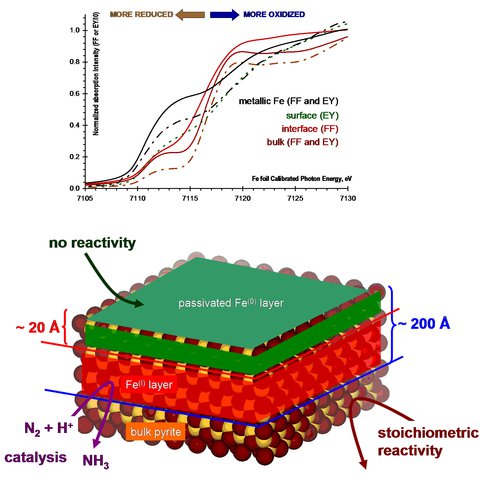
High energy (7 keV) Fe K-edge, intermediate energy (3 keV) S K-edge, and low energy 0.8 keV Fe L-edge X-ray absorption spectroscopic measurements together revealed an intriguing layered structure of the pyrite samples created in D/Ar beam surface scattering experiments. As expected upon exposure to air the sulfur depleted surface will adopt the same structure and composition as the surface of metallic iron powder. Beneath this protective, passivating layer is a novel Fe-S phase that shows spectral features consistent with formally Fe(I) oxidation state surrounded with sulfide (S2-) ligands. This layer sits on the bulk pyrite with Fe(II) and persulfide (S22-) ligands. We have collected complementary data from both the Fe and S points of view that unambiguously support the above assignments. The presence of the tentatively assigned Fe(I)2S phase next to a Fe(II)S2 is relevant to the catalytically active site of the hydrogenase enzyme with Fe(I) and Fe(II) centers linked by sulfur atoms.
Fe K-edge X-ray absorption near-edge spectra (A) and the schematic representation (B) of the layered Fe-S phases formed in pyrite surface exposure to D/Ar beam at elevated temperature
Publications
- Koll, C.J., Vance, M. & Szilagyi, R.K. (2010). Coordination Chemical Models for Pyrite Surface Sites. In Preparation.
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Timothy Minton
Project Investigator
Robert Szilagyi
Co-Investigator
Che Li
Postdoc
David Gardenghi
Doctoral Student
-
RELATED OBJECTIVES:
Objective 3.1
Sources of prebiotic materials and catalysts
Objective 3.2
Origins and evolution of functional biomolecules
Objective 3.3
Origins of energy transduction
Objective 7.1
Biosignatures to be sought in Solar System materials
Objective 7.2
Biosignatures to be sought in nearby planetary systems