2010 Annual Science Report
 Montana State University
Reporting | SEP 2009 – AUG 2010
Montana State University
Reporting | SEP 2009 – AUG 2010
Virtual Catalysis From Molecular Beam Scattering
Project Summary
Molecular beam/surface scattering experiments provide a controlled environment for modeling abiotic processes at the interface of lytho- and atmosphere. Specifically, it has been proposed that exposed rock surfaces may have played a role in modifying activated atmospheric molecules in the presence of UV radiation toward the building blocks of life. We have found that extended exposure of pyrite mineral surfaces to hydrogen atoms creates a reduced iron surface. The reduced state and the modified geometric structure of the surface iron atoms were confirmed by X-ray spectroscopy. Furthermore, this modified pyrite surface shows remarkable chemical reactivity in converting the hyperthermal beam of N2 to ammonia.
Project Progress
The molecular beam scattering experiments of the Minton laboratory allowed for progress in defining an approach to map the parameter space of experimental conditions relevant to Hadean conditions. Using various H/D beam sources, we created modified pyrite surfaces in a controlled high vacuum environment as a function of exposure time and surface temperature. The goal of these experiments is to study the activation of inert, small molecules, such as N2, H2, CO/CO2 on reactive mineral surfaces. This activation process is arguably a critical step in establishing a pool of more complex molecules such as the building blocks of life.
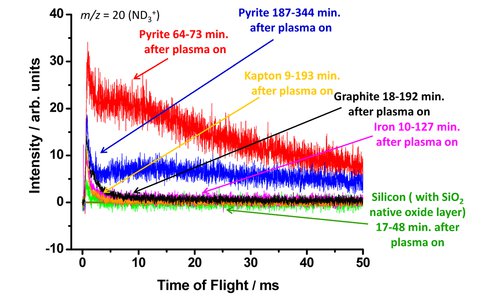
We have shown that H (or D) atoms react with a pyrite surface to produce H2S/D2S, leaving a surface enriched in reduced Fe. New experiments investigated the catalytic activity of the H/D-atom exposed surfaces at elevated temperatures by directing N2 beams at pyrite surfaces. We have observed ammonia (NH3 or ND3) using a beam containing ~6% N and ~94% N2, with kinetic energies of 5 and 10 MJ mol-1, respectively. Shorter D exposure times (1-1.5 hr) showed higher ND3 signals than those with longer exposure (3-6 hr), suggesting that a partially reduced pyrite surface has the most activity. We verified the unique reactivity of the reduced pyrite surface by conducting similar experiments with other surfaces, such as iron (Haber-Bosch catalyst for ammonia synthesis), glass (silica), polymer (Kapton), and pure carbon (graphite). All surfaces including metallic iron showed significantly less ammonia production than the reduced pyrite surface. It appears that there is a surface effect that facilitates ammonia production from the N and D atoms in the reagent beams simply by providing a medium for them to interact. However, the magnitude and nature of the ammonia signals from the reduced pyrite also suggest strong catalytic activity. We are currently investigating the mechanism of the N N triple bond activation on reduced pyrite surfaces.
Comparison of ND3+ product formation from hyperthermal N/N2 beam scattering on D/Ar beam modified pyrite surfaces
Publications
- Che, L., Gardenghi, D.J., Szilagyi, R.K. & Minton, T.K. (2010). Formation of Reduced Fe-S Phase upon Exposure of Pyrite to Atomic Hydrogen at Elevated Temperatures. In Preparation.
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Timothy Minton
Project Investigator
Robert Szilagyi
Co-Investigator
Che Li
Postdoc
David Gardenghi
Doctoral Student
-
RELATED OBJECTIVES:
Objective 3.1
Sources of prebiotic materials and catalysts
Objective 3.2
Origins and evolution of functional biomolecules
Objective 3.3
Origins of energy transduction
Objective 7.1
Biosignatures to be sought in Solar System materials
Objective 7.2
Biosignatures to be sought in nearby planetary systems