2010 Annual Science Report
 Montana State University
Reporting | SEP 2009 – AUG 2010
Montana State University
Reporting | SEP 2009 – AUG 2010
Biomineralization
Project Summary
Minerals formed by biological systems such as bones, teeth, and seashells can be used to identify the organism from which they came. Thus these mineral structures are unique biosignatures. Our efforts are focused on trying to identify the key underlying factors that are responsible for the subtle differences in the structure of these biominerals imparted by the organic biological components of the organism. We therefore study the interface between the mineral and the organic components with a particular focus on very early events in the mineral growth process.
Project Progress
Macroscopic biominerals (bones, teeth, seashells, etc.) can be a very direct signature for the presence of life. The formation of these macroscopic materials ultimately begins at the nanoscale, where the nucelation and initial growth of inorganic materials is controlled by a protein template. As part of the ABRC biomineralization thrust a collaboration (Douglas and Parise groups) has developed to investigate how the hard-soft interface (organic-mineral) influence the characteristics of the biomineral. This influence occurs at the gross level of particle morphology but also at more subtle levels of mineral orientation, polymorph selection, and influences to the electronic structure of the mineral. Molecular processes of metal-ligand cluster assembly in biology (metal sulfide and metal oxide clusters) may have parallels in the early events in biomineral nucleation. A major focus of research in this thrust area is on precisely how the initial stages of inorganic mineral formation occur at protein surfaces. We have applied a variety of physical techniques including mass spectrometry, high-energy X-ray diffraction, theoretical simulations, analytical ultracentrifugation and light scattering to probe this process of protein-directed mineral formation and growth. By using techniques that can address this process at a variety of spatial and temporal scales, we hope to understand how biology has combined the versatility and evolvability of soft polymeric materials with the often-superior material properties of inorganic materials to enable the emergence of complex life. We have strong evidence that the structural and electronic properties of protein-templated nanoparticles are significantly different from materials with the same composition synthesized without a protein template.
In particular, total X-ray scattering studies have shown that Mn oxide nanoparticles grown inside a hollow protein cage (ferritin) adopt a structure similar to chalcophanite, a layered manganese oxyhydroxide, while nanoparticles grown in the absence of protein show an inverse spinel structure similar to hausmannite. Extensive studies of Fe oxide nanoparticles have shown that materials grown within a protein cage show smaller crystalline domains than their bulk counterparts, suggesting that crystallinity may be limited by the presence of multiple adjacent nucleation sites.
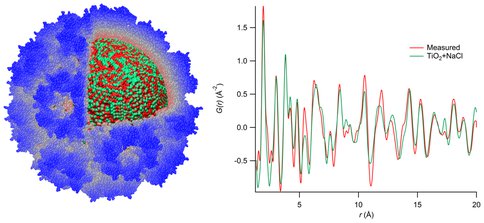
Bio-inspired mineralization methods were used to synthesize TiO2 (anatase) nanoparticles inside the virus capsid from cowpea chlorotic mottle virus (CCMV). These mineral-protein nanocomposites were analyzed using total X-ray scattering, yielding the real-space pair distribution function shown on the right. Like many of our protein-inorganic composite materials, these materials exhibit a crystalline domain size much smaller than the capsid’s inner diameter, suggesting that the structure of the inorganic phase is influenced more strongly by the nucleation and growth process than by the size constraints imposed by the confined environment. Protein-inorganic nanocomposites such as these could be a valuable model system for understanding biomineralized structures such as the magnetosome, a potential “nano-fossil” of biogenic magnetite produced by magnetotactic bacteria
Publications
-
Jolley, C. C., & Douglas, T. (2010). Ion Accumulation in a Protein Nanocage: Finding Noisy Temporal Sequences Using a Genetic Algorithm. Biophysical Journal, 99(10), 3385–3393. doi:10.1016/j.bpj.2010.09.001
-
Jolley, C. C., Uchida, M., Reichhardt, C., Harrington, R., Kang, S., Klem, M. T., … Douglas, T. (2010). Size and Crystallinity in Protein-Templated Inorganic Nanoparticles. Chem. Mater., 22(16), 4612–4618. doi:10.1021/cm100657w
-
Jolley, C., Klem, M., Harrington, R., Parise, J., & Douglas, T. (2011). Structure and photoelectrochemistry of a virus capsid–TiO 2 nanocomposite. Nanoscale, 3(3), 1004–1007. doi:10.1039/c0nr00378f
-
Klem, M. T., Young, M., & Douglas, T. (2010). Biomimetic synthesis of photoactive α-Fe 2 O 3 templated by the hyperthermophilic ferritin from Pyrococus furiosus. J. Mater. Chem., 20(1), 65–67. doi:10.1039/b918620d
- Klem, M., Young, M. & Douglas, T. (2008). Biomimetic Synthesis of b -TiO 2 Inside a Viral Capsid. Chem. Mater, 22: 4612-4618.
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
John Parise
Co-Investigator
Richard Harrington
Postdoc
Craig Jolley
Postdoc
Michael Klem
Postdoc
-
RELATED OBJECTIVES:
Objective 7.1
Biosignatures to be sought in Solar System materials
Objective 7.2
Biosignatures to be sought in nearby planetary systems