2010 Annual Science Report
 NASA Jet Propulsion Laboratory - Titan
Reporting | SEP 2009 – AUG 2010
NASA Jet Propulsion Laboratory - Titan
Reporting | SEP 2009 – AUG 2010
Task 3.4 Tholin Chemical Analysis
Project Summary
New techniques need to be developed to characterize the chemical composition of tholins at the molecular structural level.
Project Progress
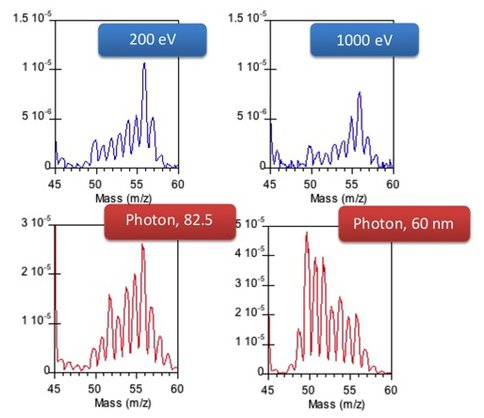
Co-Investigator Mark Smith and colleagues have preliminary results on gas phase neutral products from fast electron exposure that indicate that electron stimulated chemistry occurs along similar pathways as that from energetic photon absorption (λ < 100 nm) but with a significant fraction following channels not allowed from photoexcitation. Data suggest that the product distribution at high energies is representative of three components. The first two represent a) the predominant ionization induced reaction products (at λ ≈ 60 nm) and b) the collision induced neutral fragment induced products seen with longer wavelength EUV (90 nm) exposure, consistent with observations at the Advanced Light Source synchrotron beamline (Lawrence Berkeley National Laboratory). However, the apparent third component suggests chemistry induced by reactive intermediates, perhaps via triplet or higher spin state manifolds, not easily produced under the stricter selection rules of photon-induced processes. An indication of these observations is shown in Figure 1.
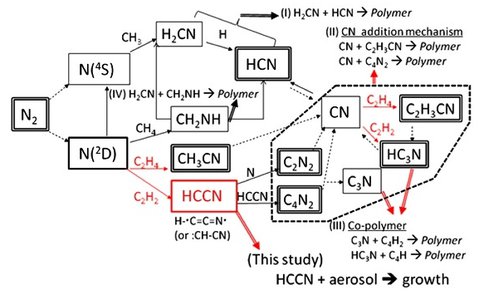
Smith et al. have completed an investigation of photolytic fixation of nitrogen into organic molecules stimulated by light at wavelengths below 100 nm. This has led to a proposed mechanism whereby atomic nitrogen, from molecular nitrogen ion-electron recombination and direct nitrogen photodissociation, reacts directly with unsaturated organics on haze aerosol surfaces. These direct surface reactions can lead to rapid loss of key reactive intermediates under the binary collision pressure regime in Titan’s ionosophere. Thus more work is needed to improve chemical reaction models to explicitly include particulate surface chemistry. See Figure 2.
Work also has focused on the vexing problems associated with tholin—plasma produced aerosol material from methane/nitrogen—separation.
Using the biomedical/proteomics separations group at University of Arizona, we renewed efforts to find suitable methods for separations of these materials minimizing chemical alteration and loss. The use of columns optimized for protein separation led to some promising results demonstrating resolution of some light (<200 amu) components of the tholin. However, the profound reactivity and polarity of the nascent tholin material presents significant problems to conventional high sensitivity chromatographic methods.
A variety of solvent systems and solid supports (silica gel, Kieselguhr and cellulose) in 1 dimensional thin layer chromatography were explored using conventional degradative detection. Again, some degree of separation could be seen, but certainly nothing that obviously would simplify definitive characterization. The use of laser desorption mass spectrometry directly from the resolved chromatographic plates was explored, but for reasons which remain unclear, could not detect significant organics leaving the plate.
Before significant progress could be made on separations of tholin materials, it is necessary to have some detailed picture of the chemical structural components within tholins. With this, passivation chemistries can be explored to present an inert chromatographable tholin descendent but in a way where the structural monitor could allow working backward from the resolved derivatives to original composition.
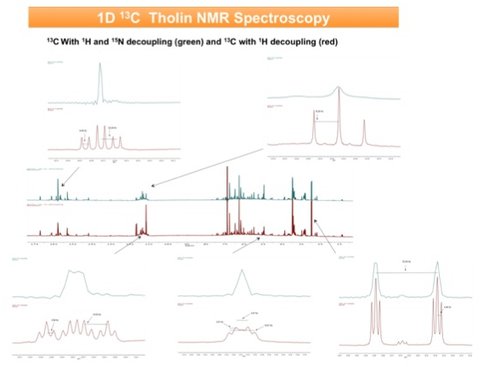
In addition there has been significant advancement into the study of the chemical structure or functional group inventory of organic aerosol haze analogues. Using fully 13C and 15N labeled reagents, we have now prepared plasma induced tholins as well as 60 nm (N2 photoionization regime) and 82.5 nm (dissociative N2 region) at the ALS. In addition, we have prepared all isotopomers of poly-HCN for comparative structure studies. We have obtained H, C and N 1D spectra of the plasma tholin and poly-HCN and 2-D H-C, C-N and N-H spectra of the poly-HCN and 2D H-C spectra of the tholin. The N spectra of the tholin and all of the spectra for the EUV photolytic aerosols await installation of an NMR cold probe to significantly improve sensitivity. An example 2D (15N-H) spectrum of poly-HCN is shown in Figure 3. Figure 4 shows the result of a proton/nitrogen decoupling experiment for the 13C spectrum of plasma tholin. This surprising spectrum suggests a predominance of key structures in these tholin materials (rather than a statistical soup) and promises to deliver key structural identification in future work.
Figure 1. Electron stimulated (200 and 1000 eV electron kinetic energy) product distributions in 5% CH4/N2 mixtures as revealed by low resolution mass spectrometry compared with EUV stimulated results.
Figure 2. Chemical reaction scheme summarizing nitrogen fixation pathways in Titans upper atmosphere, highlighting critical gas phase removal channels induced by organic particulates.
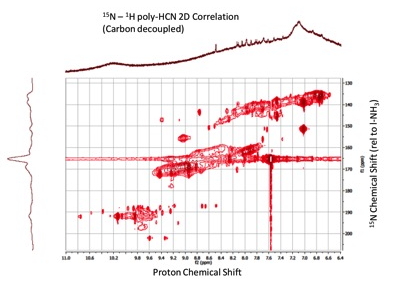
Figure 3. Two dimensional solution phase NMR spectrum of H13C15N in d6-DMSO. The 2D experiment is capable of resolving proton spectral structure not possible from 1D investigations, as seen from the distinct splitting near proton shifts of 9.5 ppm.
Figure 4. Preliminary solution phase (in DMSO) 1D 13C NMR spectra of fully 13C and 15N labeled plasma tholin haze analogue. The blue traces are obtained while saturating the proton and nitrogen spins leading to multiplets only for carbon atoms with carbon nearest neighbors. The lack of significant splitting in these H/N decoupled spectra suggest little direct C-C bonding. The red traces are obtained while only saturating the H nuclei and thus reveal 15N induced 13C coupling. Here we see a large variety of C signals split by multiple N nearest neighbors. Full analysis is in progress.
-
PROJECT INVESTIGATORS:
-
RELATED OBJECTIVES:
Objective 1.1
Formation and evolution of habitable planets.
Objective 2.2
Outer Solar System exploration
Objective 3.1
Sources of prebiotic materials and catalysts
Objective 3.2
Origins and evolution of functional biomolecules
Objective 3.3
Origins of energy transduction