2010 Annual Science Report
 NASA Jet Propulsion Laboratory - Titan
Reporting | SEP 2009 – AUG 2010
NASA Jet Propulsion Laboratory - Titan
Reporting | SEP 2009 – AUG 2010
Task 3.3.1 Solubility of Organics in Methane
Project Summary
The first step in understanding what chemistry might occur in the Titan lakes requires understanding the degree to which organics can actually dissolve in liquid hydrocarbons.
Project Progress
In the past year, Co-Investigator Robert Hodyss performed measurements of the solubility of rare gases in liquid ethane using the quadrupole mass spectrometer system that was developed in the first year of this work. Briefly, a quadrupole mass spectrometer (SRS 200) in a small vacuum chamber is used to sample liquids through a capillary into the mass spectrometer. Liquid drawn into the capillary will rapidly vaporize and be analyzed with the mass spectrometer. Saturated solutions of rare gas are made by first condensing ethane in a LN2 cooled, temperature controlled dewar, then bubbling the rare gas of interest through the solution until saturation is reached. A mass spectrum of a representative mixture of argon in liquid ethane is shown in Figure 1. By monitoring the selected peaks in the mass spectrum, we can observe the solution as it reaches saturation (Figure 2). Final concentrations are calculated based on the sensitivity of the mass spectrometer to the particular species. The solubility of argon in liquid ethane at 94 K was determined to be 4.6 × 10-2 mole fraction, consistent with theoretical values.
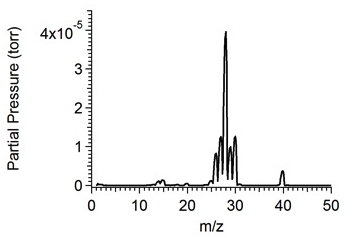
Figure 1. Mass spectrum of a saturated solution of argon in liquid ethane at 94 K. The peak at 40 m/z is due to Ar, and the group of peaks between 26 and 30 m/z are ethane.
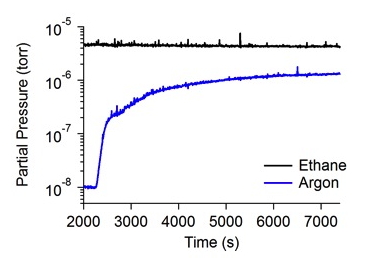
Figure 2. Partial pressure of ethane and argon with time, showing saturation of the liquid ethane sample with argon.
After the solubilities of the Kr, Xe, and N2 are determined, he will begin to use this system to determine the solubilities of small organic species. He will begin with the small aromatics and nitriles such as benzene and acetonitrile, which are expected to have relatively large solubilities in liquid methane/ethane. This method of sampling and analysis of liquid hydrocarbon solutions also could be easily adapted for in situ Titan missions with the goal of sampling Titan’s lakes.
-
PROJECT INVESTIGATORS:
-
RELATED OBJECTIVES:
Objective 1.1
Formation and evolution of habitable planets.
Objective 2.2
Outer Solar System exploration
Objective 3.1
Sources of prebiotic materials and catalysts
Objective 3.2
Origins and evolution of functional biomolecules
Objective 3.3
Origins of energy transduction
