2010 Annual Science Report
 NASA Jet Propulsion Laboratory - Titan
Reporting | SEP 2009 – AUG 2010
NASA Jet Propulsion Laboratory - Titan
Reporting | SEP 2009 – AUG 2010
Task 2.2.2.1 Ultraviolet/infrared Spectroscopy of Ice Films
Project Summary
These experiments explore to what extent long wavelength photons, the main solar radiation penetrating deep into the Titan atmosphere, can initiate chemical reactions in Titan atmospheric ices.
Project Progress
Co-Investigator Murthy Gudipati working with Postdoctoral Fellows Ronen Jacovi and Antii Lignell, and Collaborator Isabelle Couturier carried out synthesis of a Titan atmospheric molecule C4N2 (dicyanoacetylene) in the newly established TOAST (Titan Organic Aerosol Synthesis and Spectroscopy) laboratory at the Jet Propulsion Laboratory, as shown in Figure 1. Because these molecules are highly reactive, it was necessary to establish the TOAST laboratory through JPL’s internal funding. Now they are in a position to synthesize these molecules, store them at -80°C and use them to prepare cryogenic (<100 K) aerosol films for further studies relevant for the Titan NAI.
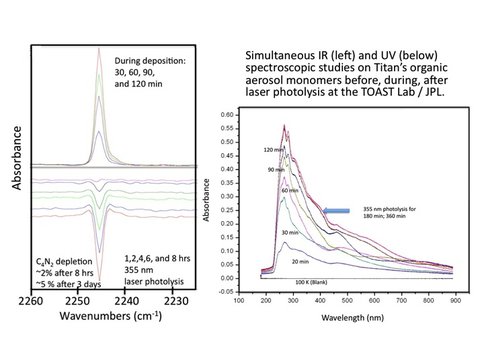
They have successfully synthesized the first targeted Titan molecule C4N2 (dicyanoacetylene) and purified it for spectroscopic and photochemical studies. Subsequently, they prepared very thin (< 500 nm) solid films of this molecule at 100 K, temperature that is close to Titan’s atmospheric aerosol temperature. The laboratory is also uniquely equipped with simultaneous UV and IR spectroscopic studies. They obtained UV and IR spectra of C4N2 simultaneously, which match well with the literature values. Subsequently, they conducted photochemistry experiments with various visible and UV lasers that are defocused to avoid multiphoton processes at wavelengths: 532 nm, 355 nm, and 266 nm. The preliminary data indicates that no photochemistry occurs at 532 nm. However, at 355 nm, which is a relatively longer wavelength than the strong absorption of C4N2 (Figure 2), they clearly see depletion of monomer absorption spectrum and increase in broad and structureless absorption, which is likely due to the polymerized C4N2, similar to the formation of atmospheric aerosols in Titan’s atmosphere. They are in the process of evaluating this spectroscopic data for publication. Thus, so far the work indicates that photochemical processes can and will occur at lower altitudes in Titan’s atmosphere where only longer wavelength solar radiation can penetrate through the atmosphere.

Figure 1: The TOAST laboratory of Dr. Gudipati at JPL, where complex organic precursor molecules of relevance to Titan are synthesized and transferred directly to the cryogenic chamber for photochemical and spectroscopic studies.
Figure 2: Simultaneous IR and UV spectroscopy of Titan’s atmospheric aerosol monomer C4N2 at 100 K. While a clear depletion of the 2244 cm-1 band of the monomer is seen during the photolysis of the cryogenic solid, an increase in the UV-VIS absorbance from 300 – 700 nm is seen in the UV-VIS spectrum upon laser photolysis at 355 nm. The increase in the absorbance is most likely due to increase in the scattering properties of the solid during photopolymerization.
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Antti Lignell
Collaborator
Ronen Jacovi
Postdoc
Isabelle Couturier
Unspecified Role
-
RELATED OBJECTIVES:
Objective 1.1
Formation and evolution of habitable planets.
Objective 2.2
Outer Solar System exploration
Objective 3.1
Sources of prebiotic materials and catalysts
Objective 3.2
Origins and evolution of functional biomolecules
Objective 3.3
Origins of energy transduction