2010 Annual Science Report
 NASA Jet Propulsion Laboratory - Icy Worlds
Reporting | SEP 2009 – AUG 2010
NASA Jet Propulsion Laboratory - Icy Worlds
Reporting | SEP 2009 – AUG 2010
Survivability of Icy Worlds
Project Summary
As part of our Survivability of icy Worlds investigation, we examine the similarities and differences between the abiotic chemistry of planetary ices irradiated with ultraviolet photons (UV), electrons, and ions, and the chemistry of biomolecules exposed to similar conditions. Can the chemical products resulting from these two scenarios be distinguished? Can viable microbes persist after exposure to such conditions? These are motivating questions for our investigation.
Project Progress
Survivability on icy Worlds investigation examines the survivability of biological compounds under simulated icy world surface conditions, and compares the degradation products to abiotically synthesized compounds resulting from the radiation chemistry on icy worlds.
Summary of Objectives and Research
Investigation 2 focuses on survivability. As part of our Survivability investigation, we examine the similarities and differences between the abiotic chemistry of planetary ices irradiated with ultraviolet photons (UV), electrons, and ions, and the chemistry of biomolecules exposed to similar conditions. Can the chemical products resulting from these two scenarios be distinguished? Can viable microbes persist after exposure to such conditions? These are motivating questions for our investigation.
Project Progress
Co-Investigator Murthy Gudipati and his group continued studying the photodegradation of PAH molecules in ices using UV radiation and electrons. In particular, they found that electrons (up to 2 keV studied here) can damage organic molecules far deeper in ice that what models predict, as shown in Figure 2.1 (bottom), where the laboratory data is well reproduced with simple empirical models involving sigmoidal penetration depth profiles for electrons. Organic probe molecules (Pyrene in this case) are completely destroyed after electron irradiation, no significant UV absorption due to any new product could be detected as seen in the UV-VIS absorption spectrum in Figure 2.1 (top), indicating that the PAHs are not only ionized in the first step, but then also degraded through consecutive radiation induced chemistry.
Gudipati and team have also been working on obtaining MALDI mass spectroscopic data for radiation processed PAHs in ices. We built a QMS system to do selective mass filtering for further studies, as a part of a different project. Earlier studies showed that we couldn’t use the same system for understanding radiation-induced chemistry of PAHs using MALDI techniques. Hence we decided to procure a TOF-MS system this year. This system has been installed recently and has shown very high sensitivity of IR induced ice ablation and UV induced ionization of the plume molecules as shown in Figure 2.2.
Several publications are in preparation. This work has been presented at DPS, AGU, AOGS, and ACAS conferences.
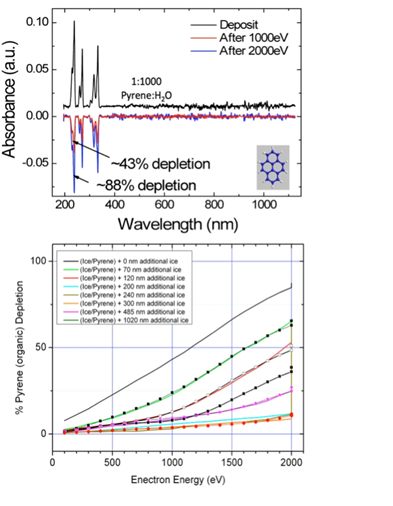
Fig.2.1. Degradation of PAH molecule pyrene upon electron irradiation (top); Empirical fit (solid lines) of laboratory data (symbols) using simple sigmoidal penetration depths of electrons and secondary radiation causing destruction of organic matter in ice.
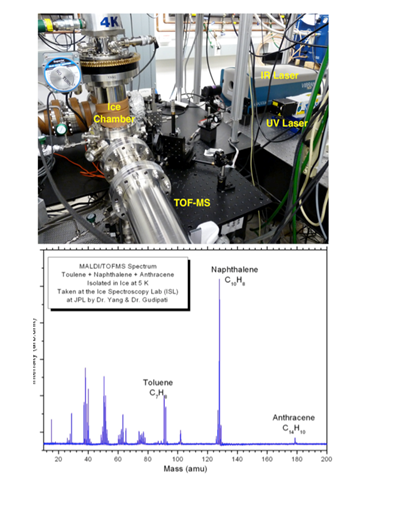
Fig. 2.2. MALDI-TOFMS system at the Ice Spectroscopy Lab(ISL) of Gudipati at JPL (top); MALDI-TOFMS spectrum of a mixture of anthracene, naphthalene, and toluene embedded in ice at 5 K.
Investigators Paul Johnson, Robert Hodyss and Isik Kanik have continued studying the photolysis of organic molecules in icy matrices relevant to solar system bodies. In this work our goal is to determine the precise chemical pathways that various molecules evolve in analog solar system ices as a function of temperature and wavelength. This involves photolysis of organics in ice, pure organics, matrix isolated organics, and organics under a layer of peroxide (to provided a source of hydroxyl radicals). Addition baseline spectra are also taken of suspected products to confirm identifications. They are measuring the photolytic lifetimes of hydrocarbons in both water and nitrogen matrices. They are also studying the photolytic chemistry of amino acid (glycine) and lipid (oleic acid and SLPC) degradation through matrix isolation experiments.
An extensive set of data have been collected on pholoysis of glycine, phenylalanine, oleic acid and SLPC. This work has involved overcoming the technical challenge of vapor deposition these large, non-volatile organics from a heated crucible without destruction of the molecule. These data are now being prepared for publication.
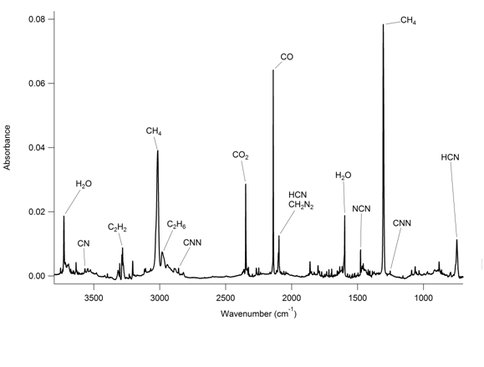
Photochemical studies of thin cryogenic ice films composed of N2 and CH4 in ratios analogous to those on the surfaces of Neptune’s largest satellite, Triton, and on Pluto have been completed. Experiments were performed using a hydrogen discharge lamp, which provides an intense source of ultraviolet light in order to elucidate the solar induced photochemistry of these icy bodies. Initial characterization via infrared spectroscopy showed that C2H6, C2H4 and C2H2 are formed by the dissociation of CH4 into H, CH2 and CH3 and the subsequent reaction of these radicals within the ice (see figure 2.3). Acetylene, C2H2,, is further photodissociated into C2. Other radical species, such as C2 C2-, CN, NCN and CNN are observed in the visible and UV regions of the spectrum. These species imply a rich chemistry based on reactions of atomic carbon with the N2 matrix. The appearance of these radical species is consistent with a chemistry based on the formation of atomic carbon and its subsequent reaction with the N2 matrix. Carbon atoms are most likely produced photolytically from C2. Ultimately, this work suggests that C2-, CN, and CNN may be found in significant quantities on the surfaces of Triton and Pluto and that new observations of these objects in the appropriate wavelength regions are warranted.
Fig. 2.3. Infrared spectrum of a 1% CH4 in N2 ice photolyzed for 550 minutes at 14 K. Prominent peaks, and those due to radical species observed in the near ultraviolet and visible are labeled. Water, carbon dioxide and carbon monoxide are present due to accretion of residual gas in the vacuum chamber.
In addition, a study of UV stimulated fluorescence of benzene, toluene and the isomers of xylene has been conducted. These results have implications for exploiting such phenomena for in situ detection of organics is solar system ices. This work is summarized in a manuscript that will be submitted to Astrobiology this fall.
In the upcoming year, the Johnson/Hodyss team will continue to explore the detailed, wavelength dependant photolysis of amino acids and lipids.
Co-I Paul Cooper of George Mason university has developed methodology to deposit hydroxy radicals into icy planetary surfaces and used these OH radicals to understand the formation of methanol in solar system ices.
The first project is a novel and innovative approach to understanding the composition of the both the surface and atmospheres of icy bodies in the outer solar system. Icy satellites are surrounded by tenuous atmospheres that contain chemical species such as H2O, OH, O2 and O. These species can deposit onto the surface, and with subsequent chemical reactions, form new chemical species. This is a radical departure from all previous work that has assumed that the composition of both the surface and atmosphere of an icy satellite is determined by the primordial composition in addition to radiolytic chemical processing. This process may be especially important at the Saturnian system due to the large neutral OH torus that exists due to cryovolcanic activity at Enceladus. Neutral OH can sweep through the system and deposit onto other icy satellites.
OH radicals were generated in a Tesla coil discharge of water vapor diluted in neon. The resulting ice is a result of depositing OH and H2O. The formation of hydrogen peroxide in the ice is strongly temperature dependent as shown by the decrease in the 2850 cm-1 band in Fig 2.4. We estimate that the OH/H2O percentage by number in the gas-phase prior to deposition in our experiments is ~12-15%. Recent modeling by Tim Cassidy (JPL) has shown that this OH abundance is comparable to the tenuous atmospheres of the icy Saturnian satellites. At 30 K we calculate there is ~ 6 % H2O2 relative to H2O, which is an order of magnitude greater than H2O2 abundances produced in irradiation experiments. At 60 K there is ~ 0.6%.
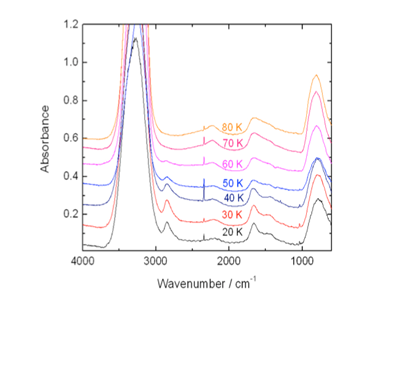
Fig. 2.4. Infrared spectra of ices produced from the discharge of 1:100 H2O/Ne gas mixtures at different deposition temperatures.
The lack of H2O2 at 80 K is likely a result of chemical destruction with depositing OH to form O2. We observe some O2 that is trapped in the ice that we have yet to quantify but there will also be some (perhaps the majority?) that is lost to the vacuum. In the case of a planetary surface this would be a source of O2 in an atmosphere. This would be applicable to both icy satellites and icy ring systems throughout the solar system.
Further work will quantify the O2 trapped in the surface and the O2 lost to the vacuum as the ice grows. This data will then be used to model surface OH reactions as an unaccounted source of O2 in tenuous atmospheres surrounding icy satellites and ring systems. Additional work will attempt to vary the OH/H2O ratio and determine the affect of surface impurities that may act as a sink for OH.
A manuscript to Ap. J. was submitted with the preliminary data showing the above results. The results have also been presented at Fall AGU in an invited speaker oral presentation and at DPS 2010.
In collaboration with colleagues at The University of Western Australia, we have shown in matrix-isolation experiments that methanol formation can occur in H2O and CH4 mixtures by the unusual O atom insertion reaction with CH4. This has been shown by using various isotopologues of water and methane. Previous reports of irradiated ices have assumed a reaction between the methyl and OH radicals. In light of these results we are preparing to begin electron-irradiation experiments to conclusively determine the reaction mechanism in an irradiated ice sample. CH4, CD4, D2O and H218O have been purchased with Icy Worlds funds and we have measured the spectra of these pure substances and some mixtures. By the end of October 2010, we will have begun irradiation experiments.
A manuscript describing the matrix-isolation data is currently in preparation. The new experimental work ice work should be completed by the end of the year with a manuscript likely to be submitted in the first quarter of 2011.
The following experiments and data analysis were also performed by co-I Strazzulla during 2010:
- Ion irradiation of methanol and methanol/CO mixture
- Determination of survival rate of Deinococcus radiodurans against ion bombardment
- Ion irradiation of sulfur bearing icy species.
Survival curves of Deinococcus radiodurans to irradiation with 200 keV protons. NC=naked cells, SST=cells mixed with sandstone grains, B=cells mixed with basalt grains, SID and SIL=cells deposited on olivine films. (Paulino-Lima, Baratta, Brucato, Cockell, Olsson-Francis, Spinella, Strazzulla, Mason, Lage, in preparation)
In 2011 we plan to study the role of energetic processing in the formation of methilformate, glycolaldheyde and other pre-biotic molecules in comets (Modica, Palumbo, Strazzulla, in preparation). The survival of bacteria mixed with grains to solar ion irradiation will be investigated.
Co-Investigators Ponce and Hand continued to study the behavior of microbial spores under energetic conditions, Investigators Kevin Hand and Adrian Ponce have installed the monodispersed spores deposition chamber. The irradiation chamber is ready for samples. Dr. Wanwan Yang, a new Caltech postdoctoral fellow, is preparing samples using chamber. We expect samples to be ready for irradiation in 2 months.
Publications
-
Garozzo, M., Fulvio, D., Kanuchova, Z., Palumbo, M. E., & Strazzulla, G. (2009). The fate of S-bearing species after ion irradiation of interstellar icy grain mantles. A&A, 509, A67. doi:10.1051/0004-6361/200913040
-
Hodyss, R., Parkinson, C. D., Johnson, P. V., Stern, J. V., Goguen, J. D., Yung, Y. L., & Kanik, I. (2009). Methanol on Enceladus. Geophysical Research Letters, 36(17), None. doi:10.1029/2009gl039336
-
Johnson, P. V., Hodyss, R., Bolser, D. K., Bhartia, R., Lane, A. L., & Kanik, I. (2011). Ultraviolet-Stimulated Fluorescence and Phosphorescence of Aromatic Hydrocarbons in Water Ice. Astrobiology, 11(2), 151–156. doi:10.1089/ast.2010.0568
-
Modica, P., & Palumbo, M. E. (2010). Formation of methyl formate after cosmic ion irradiation of icy grain mantles. A&A, 519, A22. doi:10.1051/0004-6361/201014101
-
Parent, P., Bournel, F., Lasne, J., Lacombe, S., Strazzulla, G., Gardonio, S., … Carniato, S. (2009). The irradiation of ammonia ice studied by near edge x-ray absorption spectroscopy. J. Chem. Phys., 131(15), 154308. doi:10.1063/1.3243849
-
Strazzulla, G., Garozzo, M., & Gomis, O. (2009). The origin of sulfur-bearing species on the surfaces of icy satellites. Advances in Space Research, 43(9), 1442–1445. doi:10.1016/j.asr.2009.01.007
- Cooper, P.D., Do, N. & Ammann, L.M. (2009). Preferential Oxidant Accumulation via Re-Deposition of Water Group Species on Icy Satellites. Fall AGU. San Francisco CA.
- Cooper, P.D., Do, N., Strube, A. & Ammann, L.M. (2010). Oxidant Loading of Icy Satellite Surfaces by Re-deposition and Subsequent Surface Chemistry. DPS. Pasadena CA.
- Do, N., Strube, A., Ammann, L.M. & Cooper, P.D. (2010). A Laboratory Investigation of the Chemistry of Resurfacing on Icy Bodies. Ap. J.
- Hodyss, R., Howard, H.R., Johnson, P.V., Goguen, J.D. & Kanik, I. (2010). Atomic Carbon Chemistry in Photolyzed Triton-Like Ices. Icarus.
- Hodyss, R., Kim, H.I., Bolser, D.K., Johnson, P.V., Goguen, J.D. & Kanik, I. (2010). Ultraviolet Photolysis of Lipids. Icarus.
- Hodyss, R., Parkinson, C.D., Johnson, P.V., Stern, J.V., Goguen, J.D., Yung, Y.L. & Kanik, I. (2009). Methanol on Enceladus, American Geophysical Union. American Geophysical Union (AGU). San Francisco, California, USA.
- Johnson, P.V., Hodyss, R., Howard, H.R., Goguen, J.D. & Kanik, I. (2010). Atomic Carbon Chemistry in Photolyzed Triton-like Ices. 42nd annual meeting of the Division for Planetary Sciences of the American Astronomical Society. Pasadena, CA, USA.
- Pearce, M., McKinley, A.J. & Cooper, P.D. (2010). Formation of Methanol in Argon Matrices. Chem. Phys. Phys. Chem.
- Strazzulla, G. (2010). Ion Bombardment of astrophysical ices (2010) 24th International Conference on atomic collision in solids. ICACS-24. Cracow, Poland.
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Robert Hodyss
Project Investigator
Louis Allamandola
Collaborator
Bob Carlson
Collaborator
Giovanni Strazzulla
Collaborator
-
RELATED OBJECTIVES:
Objective 2.2
Outer Solar System exploration
Objective 3.2
Origins and evolution of functional biomolecules
Objective 5.1
Environment-dependent, molecular evolution in microorganisms
Objective 5.3
Biochemical adaptation to extreme environments
Objective 7.1
Biosignatures to be sought in Solar System materials
Objective 7.2
Biosignatures to be sought in nearby planetary systems