2010 Annual Science Report
 NASA Jet Propulsion Laboratory - Icy Worlds
Reporting | SEP 2009 – AUG 2010
NASA Jet Propulsion Laboratory - Icy Worlds
Reporting | SEP 2009 – AUG 2010
Detectability of Life
Project Summary
Detectability of Life investigates the detectability of chemical and biological signatures on the surface of icy worlds, with a focus on spectroscopic techniques, and on spectral bands that are not in some way connected to photosynthesis.Detectability of life investigation has three major objectives: Detection of Life in the Laboratory, Detection of Life in the Field, and Detection of Life from Orbit.
Project Progress
Summary of Objectives and Research
How can life and biological materials be detected on the surface of icy worlds? Earth’s frozen environments can serve as important learning and testing grounds for understanding the limits and dynamics of potential life on icy worlds of the outer solar system. We are studying two different earth analog environments to better understand the diversity of life, associated functional processes, and biosignatures associated with this life in the cold. Additionally we are targeting biogeochemical processes of relevance to nutrient cycling in these environments that may give rise to biosignatures other than the molecular signatures of photosynthesis. Two different environments have been chosen to develop these objectives: the snow fields of Mt. Conness in California, and the water column and sediments of thermocarst lakes in Alaska’s North slope. Our objectives include characterizing physical and chemical characteristics, bacterial activity, and bacterial community composition of frozen and liquid water in lakes near Barrow, Alaska. This research will be used to determine the detectability of life in the field, as well as provide samples to aid other investigations focused on viability in icy habitats and detectability of life in the laboratory.
Specific objectives include:
(1) to determine the composition of the microbial community in the snow fields of Mt. Conness,
(2) to determine what evidence is there of Archaea in the water column and sediments of tundra Arctic lakes,
(3) to quantify production rates of methane gas as an evidence of possible microbial life inhabiting the sediments of Arctic lakes and (4) to identify the source of the methane, possibly psychrophilic methanogenic Archaea and the pathways by which they produce methane.
(4) Investigate the production, preservation, and detection of chemotrophic lipid biological markers in Arctic thaw lakes as an analog for life detection under Icy Worlds conditions,
(5) Generate historical, biomarker-based records of methane cycling and primary production in Arctic permafrost-bound lakes from sediment core analyses,
(6) Integrate biomarker records with parallel datasets regarding microbial ecology, water column chemistry and ice composition obtained by other team members.
(7) Couple spectroscopic and spectrometric techniques to the above measurements so as to enable in situ measurement of the chemical and molecular signatures of life in these regions.
The Astrobiology of Icy Worlds team conducted a roughly 1-month field campaign in the Barrow, Alaska region during April, 2010. Much of the successful campaign was organized and carried out by postdoctoral researchers and graduate students working with the Co-investigators. Along with our fieldwork we visited a school in remote Atqasuk, Alaska and gave a heavily attended public talk in Barrow.
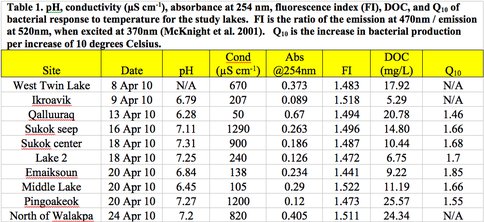
Limnological analyses were largely conducted by our Montana State Univeristy team. Water and ice samples were collected in April 2010 and the characterization of ice nutrients and bacteria are currently under way at the sub-zero ice core facility at MSU. Only the lake North of Walakpa had more than 1 m of liquid water beneath the ice cover during our period of collection. The ice covers were 1 to 1.5 m thick on most lakes, with the exception of Lake Qalluuraq, which had an ice cover of < 0.7 m. All the sites had water temperatures near 0°C throughout the water column and pH ranged from 6.28 to 7.31 (Figure 3.1).
Fig. 3.1.
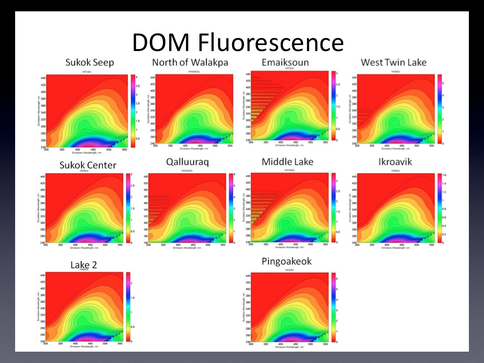
Lakes Sukok, Pingoakeok, West Twin, and North of Walakpa all had conductivity >600 μS cm-1 while Lake 2, Ikraovik, Emaiksoun, Middle, and Qalluuraq had conductivity <250 μS cm-1. DOC concentrations were measured using high temperature oxidation with a Shimadzu TOC analyzer and ranged from 5.29 to 25.57 mg/L. The absorbance at 254 nm, which provides an indication of aromatic DOC concentration ranged from 0.09 in Ikroavik to 0.67 in Qalluuraq lake indicating an over five-fold variation in aromatic DOC among the study sites. Three dimensional emission-excitation matrices (EEMs) from a Fluoromax 4 revealed differences in the quality of DOC among the lakes (Figure 3.2).
Fig. 3.2. Excitation-emission martices for the study lakes. Y-axis denotes excitation wavelength from 240 to 440 nm; x-axis denotes emission wavelength from 200 to 550 nm. Scale bar represents relative fluorescence.
The ratio of emission at 470 nm to emission at 520 nm, when excited at 370 nm can be used as a fluorescence index (FI) that reveals the source of fluorescing organic matter in the sample (McKnight et al. 2001). Ratios close to 1.1 indicates a terrestrial origin of the DOC whereas FIs closer to 1.9 indicates that the DOC is microbial in nature. FIs in our samples were all near 1.5 indicating that the DOC is derived equally from both terrestrial and microbial sources (Figure 3.1).
Bacterial activity was measured as both leucine incorporation and methane oxidation. Optimum concentrations of leucine (20 nM) and length of incubation (2 to 6 hrs) for measuring BP have been determined and preliminary temperature incubations indicate large differences in response by bacterial communities in different lakes (Figure 3.1, Figure 3.3).
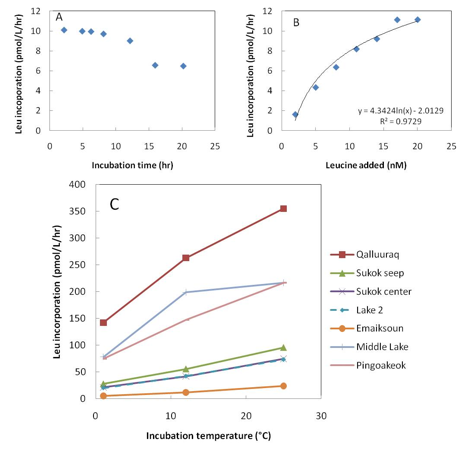
Fig. 3.3. Leucine incorporation of bacteria at the study sites. Top panels, A and B, are responses in leucine incorporation to leucine concentration and length of incubation at 20 nM leucine by bacteria from Emaiksoun Lake. The bottom panel, C, is the response to incubation temperature by bacteria at Qalluuraq, Sukok seep and center, Lake 2, Emaiksoun, Middle lake, and Pingoakeok.
All lakes showed a general increase of BP with increasing temperatures, and the highest rates were measured in samples from Qalluuraq, Middle Lake, and Pingoakeok (Figure 3.3). Sukok center had very similar rates to Lake 2, and slightly lower rates than the Sukok seep site indicating that the methane seep in Sukok may be increasing bacterial production in the immediate area. Methane samples from time-course experiments indicate methane oxidation in the water column of most lakes and were not correlated with methane concentration (Figure 3.4), suggesting that dissolved methane was at saturation levels. Ongoing analyses of bacterial community composition may identify key populations within the community and explain observed patterns of bacterial activity.
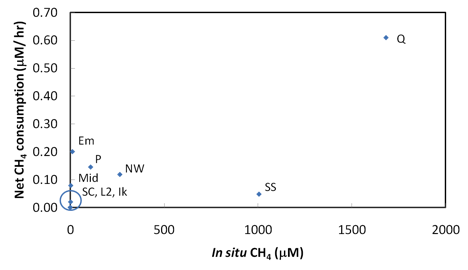
Fig. 3.4. Net methane consumption versus water column methane concentrations at West Twin (WT), Ikroavik (Ik), Qalluuraq (Q), Sukok seep (SS), Sukok center (SC), Lake 2 (L2), Emaiksoun (Em), Middle (Mid), Pingoakeok (P), North of Walakpa (NW).
Much of the microbial activity and DNA/RNA analyses for both Alaska and the Sierra snowpack is being conducted by the team at the Desert Research Institute in Reno, NV. To corroborate field based spectroscopic data, in 2009 we used (i) confocal microscopy to visualize and count algal and bacterial cells; (ii) a PCR-based approach combined with DGGE to do DNA profiling of the three divisions of life (Bacteria, Archaea and Eukarya); and (iii) we performed chemical analyses of the samples to determine dissolved inorganic nutrients, dissolved organic carbon (DOC), particulate organic carbon (POC), particulate nitrogen and chlorophyll a concentrations. The early July snow field sampled in the Mt. Conness region contained moderate levels of algal cell counts (1.1-1.8 × 103 cells mL-1). These numbers were in the range estimated by Painter and collaborators using flight-based spectroscopy (2001). Bacterial counts of DAPI-stained cells indicated that bacterial cells were more abundant than algal cells (4.1-4.5 × 103 cells mL-1), and that biomass was higher in areas where red pigmented snow was observed. The structure of the bacterial community was more complex than the structure of the eukaryal assemblage, as shown by the higher number of phylotypes detected by DNA profiling of the 16S (20-30 phylotypes per sample) and the 18S (4-17 phylotypes per sample) rRNA gene. Attempts to amplify the 16S rDNA of Archaea were negative. Though there were several unique phylotype banding patterns, there was no trend in richness across the horizontal or vertical gradients sampled. There were unique phylotypes of for both bacteria and eukarya that were observed. In addition we recently created a ribosomal RNA gene clone library for bacteria and eukarya from one sample each to get a more detailed look into the identies of life in the summer-time snow field. Also, chlorophyll a concentration (0.05-0.6 mg mL-1) correlated with numbers of algal cells throughout the vertical profile, although no correlation was found between the two variables along the horizontal transect. Likewise, the concentration of inorganic sources of nitrogen and dissolved organic carbon (DOC) varied along the horizontal transect. However, these concentrations showed a tendency to increase with depth in the vertical profile, particularly in those layers were the presence of microorganisms was shown to decline, suggesting a lack of consumption. Other nutrients, such as nitrite, phosphorous and particulate organic matter remained almost unaltered throughout the study site.
The second field program concerning life detection is taking place in the Arctic tundra lakes of the North Slope of Alaska. A number of these lakes release ebullient methane (Walter et al., 2007). This methane has three sources of methane and can be discriminated by age of production from current day – biogenic origins, to ancient in which the methane can be associated with coal beds or is associated with methane hydrates. Co-I Walter is working to characterize different lakes of the North slope in terms of their methane source using stable and 14-C isotope signatures. Lakes with biogenic methane harbor Archaea – the microorganisms that perform methanogenesis. Methane has been detected in the atmosphere of icy worlds such as Saturn’s moon Titan, and although not known if of biologic origin, it could be a feasible signature of life based on chemical rather than photosynthetic energy. The overall goals of the Murray-Group component of the Arctic Tundra Lakes program are to characterize the archaeal component and associated processes in the plankton and underlying sediments where methanogenic, or methanotrophic archaea typically reside. During a field campaign in April 2010, nine arctic lakes (West Twin Lake, Ikroavik, Qalluuraq, Sukok, White lake, Emaiksoun, Middle lake, and Pingoakeok) located around the towns of Barrow and Atqasuk, AK, were chosen to obtain samples of ice, water, and sediments. We studied the processes of methane production and consumption, prepared and inoculated media to isolate methane-producing archaea, and subsampled sediment cores (ranging from 30-90cm) to describe the microbial community. The archaea in the water column will be detected using fluorescent in situ hybridization (FISH) and using a DNA targeted approach to amplify the ribosomal RNA gene, in which we’ll sequence. We intend to characterize the archaeal component of the microbial community in the water column and the microbial community in the sediments by: (a) performing cell counts (SYBER GOLD staining), (b) performing FISH using archaea and methanogens targeted probes to quantify and identify different phyla represented© using standard (PCR-DGGE) DNA profiling techniques and high-throughput pyrosequencing.
Back in the laboratory at the Desert Research Institute (DRI), gas samples collected to determine in situ rates of methane production were determined by gas chromatography (GC). Results obtained indicated the presence of methane in lakes Ikroavik, Qalluuraq, and Sukok, with the highest concentrations detected in lakes Qalluuraq and Sukok, though no significant production was observed. The rest of the sediment plugs have been used to start enrichment cultures for methanogens in media designed for fresh water environments. To monitor cell growth the headspace of the cultures is sampled every month and methane concentration by gas chromatography is determined. This will also allow determining methane production rates in vivo. The goal is to obtain pure cultures of methanogens and other microorganisms that thrive in the sediments under given geochemical conditions. These microorganisms are expected to aid in instrumentation testing for biosignatures detection in future missions to the icy worlds.
Figure 3.5 summarizes methane concentration results from the sediment columns as a function of time.
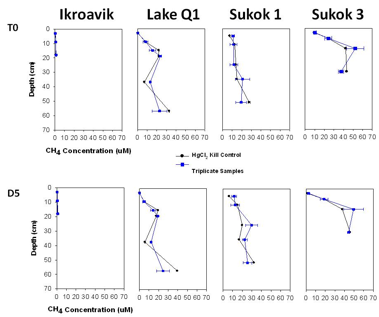
Fig. 3.5. Methane concentration of sediment samples at time 0 and day 5 of incubation.
Geochemical analyses for the Alaska fieldwork is largely being conducted by our team at the University of California, Riverside. Sediment core samples collected during fieldwork in April 2010 have all been freeze-dried and gently homogenized, completing the first step of the protocol developed for this project. Sedimentological descriptions of the cores have been compiled for communal use. Appendix I provides a methods section detailing field and laboratory techniques. Initial lipid biomarker results from the Sukok Lake seep (S1) sediment core are presented below. Intact test cores from three additional lakes remain frozen at UCR; these will be analysed prior to the April 2011 campaign as a guide to further sampling efforts.
Detailed results from our analytical methods are as follows:
i) Free (extractable) hydrocarbons released from Sukok S1 core (to a depth of 51 cm) show significant source contributions from continental plant waxes, algae and bacteria but no detectable markers unambiguously identifying archaea (methanogens or anaerobic methanotrophs). Minor amounts of ancient mature hopane and sterane biomarkers were detected, indicating a very small petroleum-like contamination, but most of the hydrocarbons were immature and derived from lake organisms (or plant matter transported into the lake) which were deposited and incorporated into sediments.
ii) Catalytic hydropyrolysis experiments were conducted on whole S1 sediments to convert functionalized lipids (alcohols, acids etc) to hydrocarbons and analysis of HyPy products for two S1 sediments supported source inputs from algae (dominated by green algae), continental plant waxes and bacteria, with again no obvious evidence for archaeal inputs. In terms of methane cycling microbes, trace amounts of 3β-methylhopanes were apparent from MRM-GC-MS analyses and these were likely derived from Type I methanotrophic bacteria which were microaerophilic (and most probably lived in the water column used molecular oxygen to oxidize methane).
iii) Unpublished results from Katey Walter Anthony indicate that the methane source emanating from Sukok S1 site is actually radiocarbon-dead (>60,000 years old) and probably derived from coalbed methane. Thus, it is not surprising that no abundant and characteristic lipid signatures from archaea could be obtained from this S1 sediment core. Initial microbiological assays conducted by Paula Matheus and Alison Murray (DRI, Nevada) also failed to find any abundant DNA signals for archaea in the SI sediment cores to 51cm depth. We conclude then that active methane generation by microbes was not significant, at least to 51 cm depth, in the Sukok S1 sediment column.
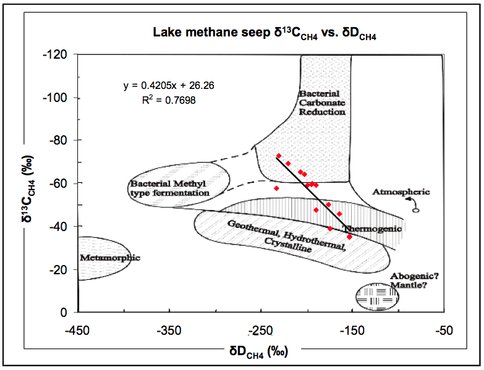
Geographic surveying of the methane bubbles trapped in ice through the North Slope of Alaska is being lead by our team at the University of Alaska, Fairbanks. Co-I Katey Walter Anthony and members from her lab group conducted ground-based field surveys of biogenic and geologic methane seeps on 36 lakes along a latitudinal transect from Barrow to the Kenai Peninsula and Gulf of Alaska. They conducted ice bubble surveys on 11 lakes on the North Slope of Alaska’s Brooks Range in the regions of Barrow and Atqasuk, 9 lakes in interior Alaska, near Fairbanks, and 13 lakes between Fairbanks and the Kenai Peninsula, including 3 additional sites in Prince William Sound and the Gulf of Alaska. Observations of ice quality were also recorded. During ground surveys they mapped over a thousand methane seeps on these lakes using GPS. They found that the majority of lakes emit methane via ebullition. From a subset of lakes they measured seepage rates and collected samples of gas for determination of mixing ratios, stable isotope values, and radiocarbon dating. Figure 3.6 and 3.7 show some of the survey activities and isotopic results.
Fig. 3.6. Some of the survey activities are shown.
Fig. 3.7. Some of the isotopic results are shown.
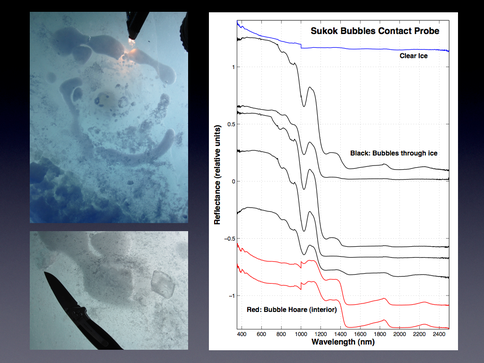
Spectroscopic characterization of the methane bubbles in the ice above the lakes and of the non-ice components within the ice has been conducted largely by our team at JPL. Near-infrared spectra were collected at several of the above mentioned lakes during the April campaign. Spectra were collected both on the surface of the lake ice and in depth profile down the ice cores drilled in the ice. Spectra from the surface revealed differences between the bubble-rich and clear ice. Though the direct detection of methane with the bubbles remains elusive, the change in ice grain size was found to serve as a useful proxy for the presence of the methane bubbles. Figure 3.8 shows a surface bubble at Sukok lake and Figure 3.9 shows the corresponding spectra from the surface.
Fig. 3.8. A surface bubble at Sukok Lake is shown.
Fig. 3.9. The corresponding spectra from the surface.
Spectra collected through depth profile in the ice were done so with an active NIR optical head mounted on a tripod and lowered down into a hole cored through the ice. Figure 3.10 shows the device in the field.
Fig. 3.10. NIR optical head mounted on a tripod is shown in the field.
Down bore-hole spectral profiles reveal layers rich in bubbles interspersed throughout the frozen water column. The spectral feature at ~1800 nm is particularly useful and diagnostic of the grain-size change observed during the surface ice calibration measurements. Figure 3.11 shows a depth profile from Lake Q in Atqasuk.
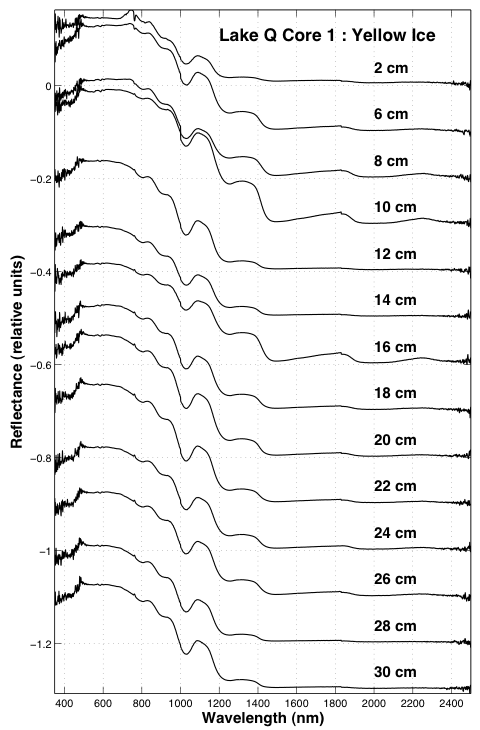
Fig. 3.11. A depth profile from Lake Q in Atqasuk is shown.
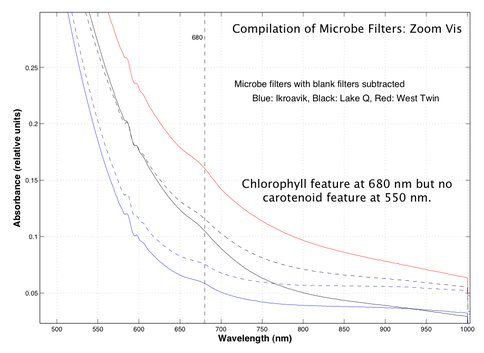
Coupled with the surface and depth mapping of the methane bubbles, spectroscopic studies of the non-ice components within the water column were conducted by using the filters from the limnological and microbial analyses. In these filters the chlorophyll signature at 680 nm can be seen, though at present this is the only spectroscopic identification of biological or non-ice material in the near-infrared. Figure 3.12 shows several spectra from these analyses.
Fig. 3.12. Results from spectroscopic studies of the non-ice components within the water column are shown.
Field Sites
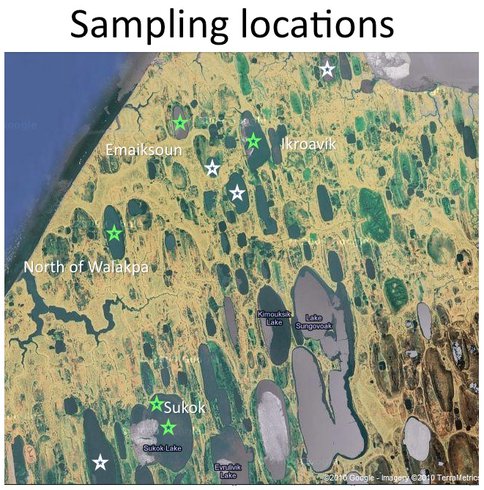
Two field sites are central to the work of this investigation: The North Slope of Alaska and the glaciers and snowpack of the Sierra Nevada Mountains. The northernmost region of Alaska is a hydrocarbon-rich permafrost environment populated by thousands of relatively small and shallow permafrost-melt lakes. The town of Barrow and Atqasuk, Alaska serve as the launching points for our field expeditions. Figure 3.13 shows some of the lakes sampled during our April 2010 field campaign.
Fig. 3.13. Some of the lakes sampled during our April 2010 field campaign.
Publications
-
Adams, H. E., Crump, B. C., & Kling, G. W. (2010). Temperature controls on aquatic bacterial production and community dynamics in arctic lakes and streams. Environmental Microbiology, 12(5), 1319–1333. doi:10.1111/j.1462-2920.2010.02176.x
-
Ball, B. A., Kominoski, J. S., Adams, H. E., Jones, S. E., Kane, E. S., Loecke, T. D., … Solomon, C. T. (2010). Direct and Terrestrial Vegetation-mediated Effects of Environmental Change on Aquatic Ecosystem Processes. BioScience, 60(8), 590–601. doi:10.1525/bio.2010.60.8.5
-
Sparks, W. B., McGrath, M., Hand, K., Ford, H. C., Geissler, P., Hough, J. H., … Turnbull, M. (2010). Hubble Space Telescope observations of Europa in and out of eclipse. International Journal of Astrobiology, 9(04), 265–271. doi:10.1017/s1473550410000285
- Adams, H.E. & Priscu, J.C. (2010). Temperature responses of polar lake bacteria.
- Cassidy, T., Coll, P., Raulin, F., Carlson, R.W., Johnson, R.E., Loeffler, M.J., Hand, K.P. & Baragiola, R.A. (2010, In Press). Radiolysis and Photolysis in Moons of the Outer Solar System: Exchange Processes Involving the Interiors’. O. Grassett.
- Clark, K., Boldt, J., Greeley, R., Hand, K.P., Jun, I., Lock, R., Pappalardo, R.T., Van Houten, T. & Yan, T. (2009). Return to Europa: Overview of the Jupiter Europa Orbiter Mission. Advances in Space Research Special Issue on Europa Lander Workshop. Moscow, Russia.
- Hand, K.P. (2010). The ocean of Europa and implications for habitability and the origin of life. Astrobiology: from simple molecules to primitive lif. Eds. V.A. Basiuk and R. Navarro-Gonzalez.
- Hand, K.P., Chyba, C.F., Priscu, J.C., Carlson, R.W. & Nealson, K.H. (2009). Astrobiology and the Potential for Life on Europa. In: Pappalardo, R., McKinnon, W. & K., K. (Eds.). In Europa. Univ. of AZ Press.
- Khurana, K.K., Kivelson, M.G., Hand, K.P. & Russell, C.T. (2009). Electromagnetic induction from Europa’s ocean and the deep interio. In: Pappalardo, R., McKinnon, W. & Khurana, K. (Eds.). Europa. Univ. of AZ Press.
- Korablev, O., Gerasimov, M., Dalton, J.B., Hand, K.P., Lebreton & J.-P., W.C. (2009). Methods and Measurements to Assess Physical and Geochemical Conditions at the Surface of Europa. Advances in Space Research Special Issue on Europa Lander Workshop. Moscow, Russia.
- Lorenz, R.D., Gleeson, D., Prieto-Ballesteros, Gomez, F., Hand, K.P. & Bulat, S. (2009). Analog Environments for a Europa Lander Mission. Advances in Space Research Special Issue on Europa Lander Workshop. Moscow, Russia.
- Raulin, F., Hand, K.P., McKay, C., Fortes, D. & Viso, M. (2010, In Press). Exobiology, Habitability, and Planetary Protection in Moons of the Outer Solar System: Exchange Processes Involving the Interiors’. In: Grassett, O. (Eds.).
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Heather Adams
Collaborator
Dan Berisford
Research Staff
Paula Matheus-Carnevali
Graduate Student
Megan Rohrssen
Graduate Student
Pamela Santibanez
Graduate Student
-
RELATED OBJECTIVES:
Objective 1.2
Indirect and direct astronomical observations of extrasolar habitable planets.
Objective 2.1
Mars exploration.
Objective 2.2
Outer Solar System exploration
Objective 4.1
Earth's early biosphere.
Objective 5.3
Biochemical adaptation to extreme environments
Objective 6.1
Effects of environmental changes on microbial ecosystems
Objective 6.2
Adaptation and evolution of life beyond Earth
Objective 7.1
Biosignatures to be sought in Solar System materials
Objective 7.2
Biosignatures to be sought in nearby planetary systems