2010 Annual Science Report
 NASA Goddard Space Flight Center
Reporting | SEP 2009 – AUG 2010
NASA Goddard Space Flight Center
Reporting | SEP 2009 – AUG 2010
The Cosmic Ice Laboratory
Project Summary
Scientists at the Cosmic Ice Laboratory with the Goddard Center for Astrobiology study the formation and stability of molecules under conditions found in outer space. During the past year, studies of amino-acid destruction were begun, projects on ethane and carbonic acid were completed, and better quantification of Titan organics became possible through our experiments. All of this work is part of the Comic Ice Laboratory’s continuing contributions toward understanding the chemistry of biologically-related molecules and chemical reactions in extraterrestrial environments.
Project Progress
Our research focuses on chemical reactions and physical properties of icy materials in extraterrestrial environments dominated by low-temperature and low-pressure conditions. Ionizing radiation sources are used to simulate space radiation conditions and to drive reactions. Infrared (IR) spectroscopy is used to monitor the chemistry in situ within a high-vacuum chamber. Although we traditionally have used 1-MeV protons to initiate ice chemistry, a H2-discharge far-UV lamp, a xenon discharge lamp, and a 10-keV electron source are also available to us. With these tools we can investigate a wide variety of interstellar, circumstellar, and solar-system chemical problems.
Over the past year we have continued our work on amino-acid chemistry, moving away from molecular formation to molecular destruction. We also have completed an extensive set of optical-constant measurements on five of the known components of Titan’s atmosphere, and have completed and published a set of measurements on the IR spectra of ethane. Finally, we also have completed and published new data on carbonic acid (H2CO3), one of the simplest, albeit elusive, products of the reaction between H2O and CO2.
Amino Acid Destruction
Previous papers from this laboratory have reported on both the radiolytic and photolytic destruction of small molecules known to be in the interstellar medium. More recently, we have turned our attention to similar measurements on amino acids as a preliminary step toward examining even larger species. With NAI support, the Goddard Center for Astrobiology funded a summer student, Mr. Jan-Luca Bell, in our laboratory from June to August of this year. Mr. Bell worked with us to measure radiolytic lifetimes of glycine at three temperatures, both in the pure solid state and in the presence of water-ice. Our long-term goal for this work is to develop a set of rate data for the radiolytic destruction of selected amino acids in various astronomical environments, such as on Titan and in the interstellar medium.
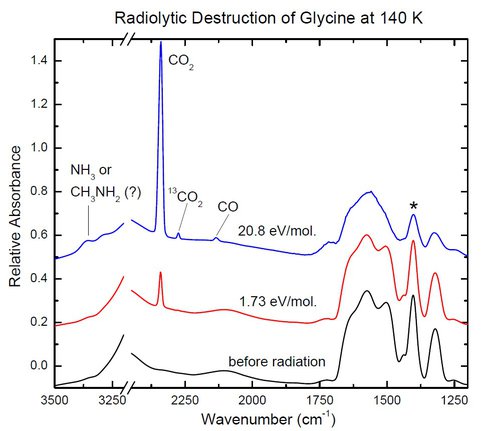
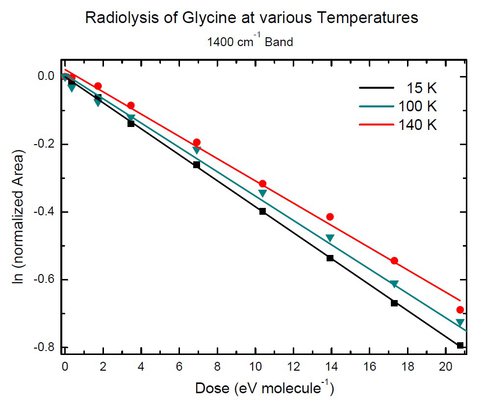
Figure 1 shows an example of the work done by our astrobiology summer student. The spectrum of glycine at the bottom is altered as the amorphous sample is bombarded with protons (E ~ 0.8 MeV). The sharp band of CO2 increases with radiation dose, as do broader features from NH3 and/or CH3NH2, the expected decomposition products of glycine: NH2CH2COOH → CH3NH2 + CO2. Measuring the area of the glycine band marked with the asterisk allowed us to quantify the rate of destruction, as shown in Figure 2.
Figure 1. Figure 1: IR spectra of glycine at 140 K showing destruction by 0.8 MeV protons and the formation of products.
Figure 2. Figure 2: First-order kinetic plots for the radiolytic destruction of glycine at three temperatures.
Similar measurements were made with glycine-water mixtures, and the results indicated a shorter glycine lifetime in the presence of H2O. Work is currently being done on the all-important timescale conversions needed to relate these results to astronomical environments. Work also is being done, in conjunction with our Exobiology grant to extend these measurements to other amino acids.
IR Optical Constants of Titan Nitriles
It is often said that Titan chemistry can serve as a model for the early-Earth’s O2-poor atmosphere. Most of the known Titan molecules were first identified by a symbiosis between spacecraft and telescopic observations and laboratory spectroscopy. To quantify spectral observations, particularly those of Titan atmospheric ices, IR optical constants are required. Unfortunately, these are often not available for the needed Titan temperatures and wavelengths. Also, in many cases the data is unavailable electronically.
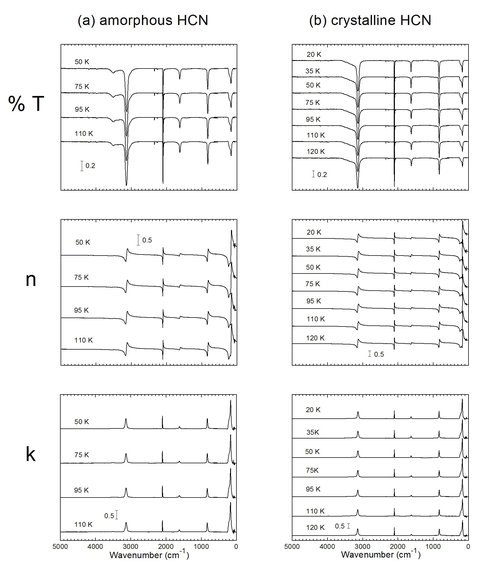
Over the past year we have leveraged NAI-GCA support with funding from NASA’s Cassini Data Analysis and Planetary Atmospheres programs to measure IR optical constants (n and k) of five molecules identified in Titan’s atmosphere: HCN, C2N2, HCCCN, CH3CN, and CH3CH2CN. A detailed paper on this work has been submitted and recently accepted by The Astrophysical Journal Supplement Series. All data will be posted by the journal and also on our lab’s web pages. An example of the wealth of data now available is shown for HCN in Figure 3, taken from our manuscript. We are now working with our collaborators to apply this data to Cassini-CIRS results.
Figure 3. Figure 3: Transmission spectra and the real (n) and imaginary (k) parts of the index of refraction of solid HCN.
Ethane Spectra and Phases
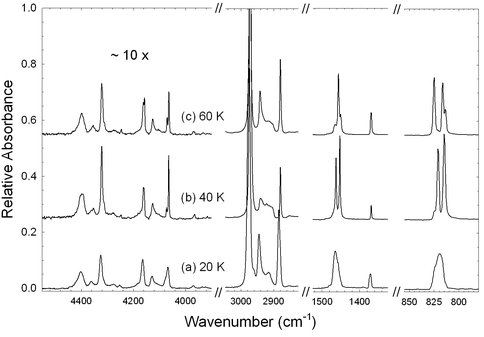
A third project of the past year was motivated by our interest in organic chemistry in the outer solar system, such as on KBOs. We completed and published a new set of measurements on three different solid phases of ethane at 10 – 70 K. Like our amino-acid studies (page 1), this work was aided by NAI-GCA support for a summer student, Ms. Lily Raines. Working with her, we were able to record near- and mid-IR spectra of amorphous, crystalline, and a little-known intermediate phase of ethane, and to tabulate relative band areas. This sets the stage for complete n and k measurements of all phases over a range of temperatures, which we are now pursuing. Figure 4 shows an example of our ethane spectra. This project has been funded through a combination of NAI-GCA support and NASA’s Outer Planets Research program.
Figure 4. Figure 4: IR spectra for (a) amorphous, (b) metastable, and© crystalline ethane offset and scaled for a common molecular column density of about 9.6 × 1017 molecules / cm2.
Carbonic Acid (H2CO3)
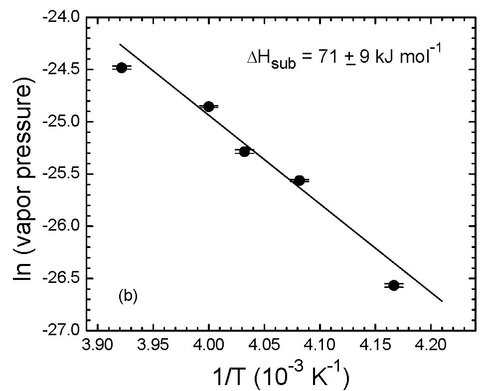
Carbonic acid is a reaction product of CO2 and H2O in essentially all biological systems, although the molecule long defied direct detection. In the past year we extended early work in our laboratory so that we now have measured this acid’s vapor pressure at 240 – 255 K, where we find that the molecule is orders of magnitude less volatile than the simpler formic and acetic acids. A paper on these findings has been accepted for publication in Icarus. The relevant Clapeyron plot is shown as Figure 5. This project has been supported by joint funding from NAI-GCA and NASA’s Mars Fundamental Research program.
Figure 5. Figure 5: The vapor pressures of H2CO3 at five temperatures. The slope of the regression line gives the heat of sublimation, ΔHsub = 71 ± 9 kJ mol−1.
Other Work
We also have continued our work on oxidants, particularly H2O2, with an eye toward Europa ices. Much of this work has been undertaken with our Europa collaborators, Robert Carlson and Paul Cooper, and a book chapter and paper have resulted. We recently have begun to explore the possibility that thermal reactions between H2O2 and organics might take place in ices even in the absence of ionizing radiation. This work is an astrobiological extension to that supported by our Planetary Geology and Geophysics award.
Publications
-
Hudson, R. L., Moore, M. H., & Raines, L. L. (2009). Ethane ices in the outer Solar System: Spectroscopy and chemistry. Icarus, 203(2), 677–680. doi:10.1016/j.icarus.2009.06.026
- Carlson, R.W., Calvin, W.N., Dalton, J.B., Hansen, G.B., Hudson, R.L., Johnson, R.E., McCord, T.B. & Moore, M.H. (2009). Europa’s Surface Composition. Chapter in Europa, University of Arizona Press.
- Hudson, R.L., Lewis, A.S., Moore, M.H., Dworkin, J.P. & Martin, J.P. (2009). Enigmatic Isovaline: Investigating the Stability, Racemization, and Formation of a Non-Biological Meteoritic Amino Acid. Chapter in Bioastronomy 2007: Modelcules, Microbes, and Extraterrestrial Life, Astronomical Society of the Pacific.
-
PROJECT INVESTIGATORS:
-
RELATED OBJECTIVES:
Objective 3.1
Sources of prebiotic materials and catalysts
Objective 3.2
Origins and evolution of functional biomolecules