2010 Annual Science Report
 NASA Goddard Space Flight Center
Reporting | SEP 2009 – AUG 2010
NASA Goddard Space Flight Center
Reporting | SEP 2009 – AUG 2010
FTT Catalysis of Organic Materials in the Solar Nebula
Project Summary
The first results from our experiments to simultaneously trap noble gases while synthesizing the macromolecular organic coating on amorphous iron silicate grains were reported at the 2010 LPSC in March and were carried out in collaboration with Drs. Charles Hohenberg and Alex Meshik at Washington University. Grain coatings were made at temperatures of 873K and 673K, yet significant quantities of Xenon and Krypton were trapped while no detectable levels of either Ar or Ne were observed above blank level. FTT synthesis in the solar nebula probably took place at much lower temperatures and the observed trapping of heavy noble gases at such high temperatures is very encouraging for the low temperature studies that we will begin this spring.
Project Progress
Progress Report: FY-10 FTT Catalysis of Organic Materials in the Solar Nebula
Over the past year we have made progress in several areas. First we reported the first results from our experiments to simultaneously trap noble gases while synthesizing the macromolecular organic coating on amorphous iron silicate grains. The results were reported at the 2010 LPSC in March and were carried out in collaboration with Drs. Charles Hohenberg and Alex Meshik at Washington University. Despite the fact that these grain coatings were made at temperatures of 873K and 673K, significant quantities of Xenon and Krypton were trapped (without isotopic fractionation) while no detectable levels of either Ar or Ne were observed above blank level. It is very likely that the majority of FTT synthesis took place at much lower temperatures and so the observed trapping of heavy noble gases at such high temperatures is very encouraging.
We included preliminary results of the noble gas trapping experiments in our last submission to the Exobiology R&A program where we proposed to analyze both the trapped gas as well as the structure and the isotopic fractionation of the FTT macromolecular coating simultaneously as a function of temperature. We will synthesize such materials from about 875K down to as low as 375K, even though these lower temperature experiments could take as long as 5 years (or more) to complete even if everything goes as planned. By comparing the trapping efficiency with the structure of the coating as a function of experimental temperature we hope to begin to constrain the conditions in the nebula where organic materials were produced. The reviewers were kind enough to recommend funding for this project, including sufficient resources in the first year of the project that will allow us to build 10 additional experimental systems so that we can carry out many experiments at different temperatures simultaneously. Money arrived at GSFC in August and we have placed orders for the new equipment as rapidly as NASA procurement rules allow. We plan to have the new systems running by the spring of 2011.
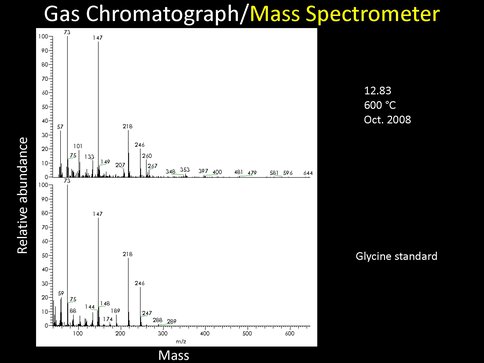
Finally, we have obtained some very intriguing preliminary results from the work of a student intern (Mickey Kopstein) who worked with our experiment and analyzed intermediate results of the synthesis in collaboration with Drs. Jason Dworkin and Jamie Elsila-Cook. The first surprise is the detection of glycine in the macromolecular coatings made at 873K: this was not expected but has spurred plans to look for the presence of additional amino acids especially in materials made at lower temperatures.
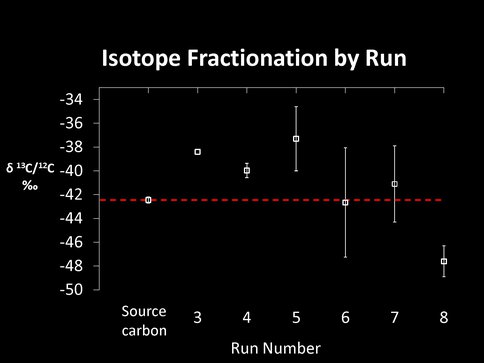
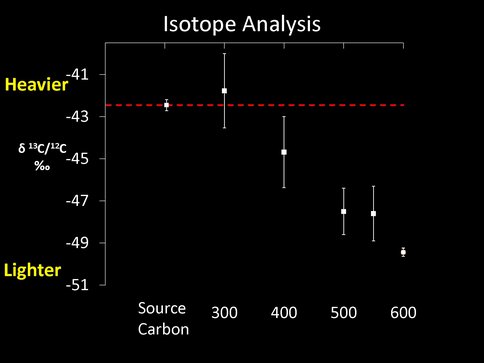
The second surprise was the detection of a slight fractionation of carbon isotopes both as a function of run number (this may be a direct proxy for time) and as a function of temperature after 15 – 20 runs. At 873K the surface residue first becomes heavier when compared to the isotopic composition of the initial CO used in the experiments (to a maximum of 6 per mil), but then becomes isotopically lighter over time. (This is exactly the opposite from what we might expect to happen – namely the buildup of isotopically heavy carbon on the FTT substrate at higher temperatures – based on experiments by Ed Anders during the 1980s.) Experiments conducted at successively lower temperatures produce less fractionated carbon in the residues, while experiments conducted at 573K might be isotopically lighter than the initial CO and more in line with our initial expectations. These experiments require additional work, especially at lower temperatures that will be possible with the new equipment, to see if the carbon isotopic composition of the residues is heavier than that of the starting CO.
Figure 1 Top. Isotopic composition of carbon in the macromolecular grain coating compared to the initial composition of the CO (source carbon) after ~15 runs as a function of temperature (in oC).
Figure 1 Bottom. Isotopic composition of the macromolecular residue compared to that of the initial CO as a function of experimental run number for experiments run at 823K.
Figure 2. Fig2 The mass spectrum of the Gas Chromatic peak released at 12.83 minutes from the analysis of the macromolecular residue produced at 873K (600oC) via FTT reactions that should be compared to the mass spectrum of a Glycine standard shown in the plot at the Bottom.
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Jamie Cook
Co-Investigator
Jason Dworkin
Co-Investigator
Joseph Nuth
Co-Investigator
-
RELATED OBJECTIVES:
Objective 3.1
Sources of prebiotic materials and catalysts
Objective 3.2
Origins and evolution of functional biomolecules