2010 Annual Science Report
 Georgia Institute of Technology
Reporting | SEP 2009 – AUG 2010
Georgia Institute of Technology
Reporting | SEP 2009 – AUG 2010
Extremophile Ribosomes
Project Summary
We will compare biochemistry and the three-dimensional structures of ribosomes from modern organisms on particular lineages of the tree of life. Extremophiles are of special interest due to their ability to thrive in environments that reminiscent of early biotic earth.
Project Progress
Purification, isolation, and identification of rotifer ribosomal proteins
We have made significant advances in purifying ribosomes from the rotifer B. calyciflorus. We modified the purification protocol and now have significant gains in overall ribosome yields. The purified proteins were analyzed on a large 1-dimensional SDS-PAGE gel, individual bands were excised and sent for mass spectrometry analysis. Most proteins identified matched proteins known from the small and large ribosomal subunits of eukaryotes. Studies are underway to further modify our preparation protocol before embarking on the purification of ribosomes from desiccated Brachionus calyciflorus. Our goal is to compare the types ribosomal proteins present in hydrated and desiccated ribosomes. Changes in ribosomal protein composition with desiccation are likely to provide insight into how ribosomes of some animals are able to tolerate drying.
Desiccation tolerance in Rotifera
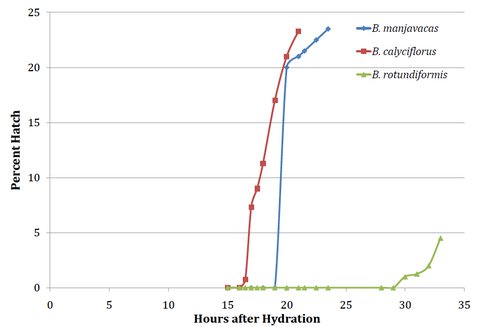
One of the biggest challenges facing eukaryote extremophiles is the loss of water leading to desiccation. Resistance to desiccation as adults, juveniles, seeds, or spores is found in species of five animal phyla and four divisions of plants. Little is understood about the biochemistry of desiccation tolerance in eukaryotes, but metabolism resuscitation via restoration of ribosome activity is likely to figure prominently in this phenomenon. The model for our investigations are animals from the phylum Rotifera which are capable of transitioning from completely desiccated to an active, swimming animal in minutes to hours (Figure 1). We are examining how their ribosomes are capable of tolerating near complete dehydration, then rehydrate and engage in translation within minutes. Our hypothesis is that specific proteins associate with ribosomes during desiccation, protecting them from damage and then dissociate upon rehydration. We want to enumerate these proteins and discover the underlying genes. In the future, this knowledge could be used to engineer desiccation tolerance into organisms that currently lack this ability.
Thermostable rotifer proteins
In monogonont rotifers, resting eggs have much greater thermotolerance than adults. We have isolated two thermostable proteins of 27 and 35 kD from resting eggs of the rotifer Brachionus calyciflorus and used mass spectrometery to determine their identity (method following previously described 1). The 27 kD protein is similar to an LEA-1B (late embryogenesis abundant) protein known from the rotifer Adineta ricciae. It plays a key role in desiccation tolerance in rotifers 2. The 35 kD protein is similar to vitellogenin, a egg yolk protein widely present in the yolk of vertebrate and invertebrate animals.
We have used the amino acid sequence obtained from these peptides to design degenerate PCR primers to amplify the corresponding genes from genomic rotifer DNA. We have cloned several PCR products of the expected size and sent them for sequencing. Once we identify the genes, we will use the yeast two-hybrid system to identify binding partners for the 27 and 35 kD proteins.
1. Warner, A. H.; Brunet, R. T.; MacRae, T. H.; Clegg, J. S., Artemin is an RNA-binding protein with high thermal stability and potential RNA chaperone activity. Archives of Biochemistry and Biophysics 2004, 424 (2), 189-200.
2. Denekamp, N. Y.; Reinhardt, R.; Kube, M.; Lubzens, E., Late embryogenesis abundant (LEA) proteins in nondesiccated, encysted, and diapausing embryos of rotifers. Biology of Reproduction 2010, 82 (4), 714-724.
Figure 1. Figure 1. Percent desiccated resting eggs hatched x hours after rehydration.
Publications
-
Denekamp, N. Y., Reinhardt, R., Kube, M., & Lubzens, E. (2009). Late Embryogenesis Abundant (LEA) Proteins in Nondesiccated, Encysted, and Diapausing Embryos of Rotifers. Biology of Reproduction, 82(4), 714–724. doi:10.1095/biolreprod.109.081091
- Warner, A.H., Brunet, R.T., MacRae, T.H. & Clegg, J.S. (2004). Artemin is an RNA-binding protein with high thermal stability and potential RNA chaperone activity. Archives of Biochemistry and Biophysics, 424(2): 189-200. doi:DOI: 10.1016/j.abb.2004.02.022
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Dana Cook-Schneider
Postdoc
Jessica Bowman
Research Staff
-
RELATED OBJECTIVES:
Objective 5.3
Biochemical adaptation to extreme environments