2010 Annual Science Report
 Carnegie Institution of Washington
Reporting | SEP 2009 – AUG 2010
Carnegie Institution of Washington
Reporting | SEP 2009 – AUG 2010
Project 4: Geochemical Steps Leading to the Origins of Life
Project Summary
The project titled “Geochemical Steps Leading to the Origins of Life” sets a out a research object focusing on exploring the natural intersection of abiological organic chemistry and the mineral world. Assuming that life emerged on Earth as a consequence of natural, geochemical, processes. We ask what did the organic landscape look like before life, how did organic-mineral surface interactions affect this landscape, and can we identify any connections between this abiotic organic Earth and the subsequent emergence of life.
Project Progress
Task 4.1 Geochemical Roots of Life
PI Cody and Post Doctoral research scientist, Shohei Ohara have continued their studies on mineral catalyzed amino acid polymerization. We have discovered that the mineral sphalerite, ZnS, promotes polymerization of moderately dilute glycine to glycyl-glycine and glycyl-glycl-glycine well above thermodynamically predicted values. Sphalerite is a commonly occurring phase in massive sulfide ore-bodies, that are found along deep oceanic spreading centers and that form through extensive hydrothermal water-rock interactions. Over the past year this project has been lead by Ohara. Based on a large number of experiments, including variable temperature kinetics and in situ monitoring via ATR FTIR, we find that sphalerite enhances the formation of simple peptides through surface absorption of glycine that increases the effective molarity by approximately an order of magnitude. The magnitude of the enhancement scales inversely with temperature, thus sulfide enhanced polymerization of amino-acids is most important at moderate to low temperatures. The results of this study were presented at the 2010 Astrobiology conference. A manuscript detailing the experiments and results is in preparation.
PI Cody continues to explore the topology of the abiotic small molecule reaction network. Working with post Doctoral Researcher Ohara, they explored the potential for the formation of uracil from fumaric acid via the addition of urea under hydrothermal conditions at elevated pressures. In the course of this investigation, the effect of pressure on the rate of decarboxylation of orotate to uracil was also explored. In modern biology the enzyme orotidine monophosphate decarboxylase is noted to be one of the most catalytically proficient enzymes, enhancing the rate of conversion of orotidine monophosphate to uridine monophosate by 17 orders of magnitude relative to same reaction in the absence of the enzyme. Our reactions at elevated temperature and pressure reveal a large positive activation volume, strongly suggesting that the reaction sans-enzyme proceeds through a free radical pathway. Intriguingly, we also observe that both uracil and orotic acid are unstable at high pressure, decomposing through a retro-Aldol reaction preceded by H2O addition. During the summer of 2010, experiments designed to refine the ultimate potential for the synthesis of uracil under hydrothermal conditions were performed by Rachel Maxwell as the focus of her summer intern research in the Cody Laboratory. Results of these studies were presented at the 2010 Origins of Life Gordon Research Conference and a manuscript is in preparation for publication.
Task 4.2 Prebiotic Molecular Selection and Organization
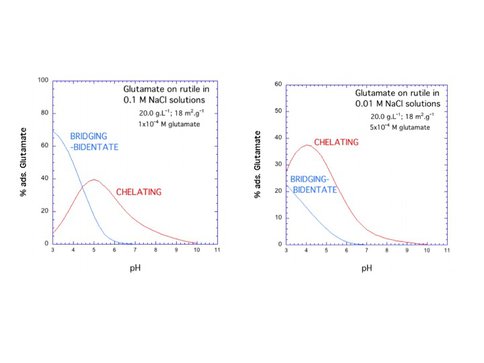
A key objective of our program is to explore mechanisms by which prebiotic molecules may have been selected and concentrated in plausible geochemical environments, especially on mineral surfaces. CoIs Hazen and Sverjenski and ORAU NAI Postdoctoral researcher Cleaves in collaboration with other colleagues have obtained fundamental data on speciation and coordination chemistry of amino acids on mineral surfaces by studies of glutamic and aspartic acid on rutile as functions of pH, ionic strength, and ligand-to-solid ratio using potentiometric titration and batch adsorption experiments. We integrated data with a new surface complexation model that enables insights into the number of inner-sphere attachment points for the amino acids at the surface. These studies are the first such comprehensive study of amino acid interactions with minerals in electrolyte solutions.
These two different types of experimental data (together with ionic strength dependence data not shown) provide strong constraints on the stoichiometry of the adsorption reactions. The surface complexation model required at least two reaction stoichiometries:
4>TiOH + H+ HGlu- = > Ti2(>TiOH)2 Glu + 2H2O
and >Ti(OH)2 + H+ + HGlu- = > Ti(OH2+)Glu- + H2O
where the release of two and one water molecule respectively indicates the number of inner-sphere attachments for each surface glutamate species. These reactions can be interpreted in terms of two surface species, where the calculated proportions of the two surface glutamate species vary strongly.
Titration and adsorption data for aspartate on rutile are similar to those for glutamate; however, our interpretation of the second surface species corresponds to an outer-sphere attachment according to the reaction: TiOH + H+ + HAsp- = > TiOH2+ _ HAsp-
Glutamate and aspartate species are consistent with ATR-FTIR spectroscopic results for aspartate on amorphous TiO2. Aspartate surface complexation model also correctly predicts a decrease of the isoelectric point with increased aspartate concentration, consistent with studies of the aspartate-anatase system. Important conclusions of our multi-technique study include:
(1) Amino acids form inner sphere (chemisorbed) complexes with Ti-O bonds – a finding that confirms conclusions of our earlier work on Asp-calcite interactions, and points to a possible role of mineral surfaces in selecting and protecting key prebiotic molecules.
(2) Multiple competing amino acid surface species occur, and the relative proportions of these vary significantly with pH and solute concentration.
(3) Both glutamate and aspartate adsorb so strongly that they can lower the zero point of charge of a mineral significantly, thus raising the intriguing possibility of cooperative adsorption of positively-charged amino acids such as lysine or arginine.
Additional studies of lysine, arginine, phenylalanine and L-dopa on rutile have also been completed and manuscripts are in preparation.
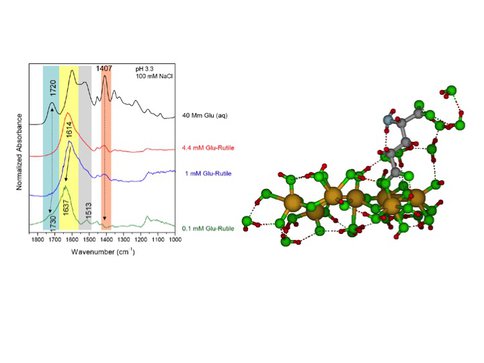
Building on batch adsorption and titration experiments, CoIs Hazen and Sverjensky also measured ATR-FTIR spectra for glutamate and aspartate under a range of conditions. With decreasing glutamate concentration from 4.4 to 0.1 mM, the 1,407cm-1 peak disappears and a new peak at 1,730 cm-1 grows. These data indicate the existence of at least two surface complexes, as well as direct involvement of carboxyl groups. Using quantum chemical calculations of synthetic IR spectra for molecular attachments we can assign the IR peaks and integrate the spectroscopic, molecular, titration, batch and surface complexation studies (see below).
CoI Sverjensky continues to perform surface complexation modeling to integrate published adsorption data for glutamate on HFO with in situ ATR-FTIR studies of glutamate speciation on amorphous TiO2. Glutamate adsorbs on HFO at low pH as bidentate-binuclear and chelating-monodentate species attached to the surface through the carboxylate functional groups. However, at high surface coverages, glutamate adsorbs as a monovalent anion chelated to the surface by the distal carboxylate. The α-carboxylate and amine groups are free to interact above the surface – results that echo our previous studies and point to general principles of molecular binding on oxide surfaces.
CoI Cleaves surveyed comparative adsorption of nucleic acid components on rutile at pH 6.7 and 0.1 M NaCl as functions of surface loading. Adsorption affinity varies significantly, with ribonucleotides > deoxyribonucleotides > ribonucleosides > deoxy-ribonucleosides > free nitrogenous bases, suggesting a role for both sugar and phosphate groups in adsorption. The base substituent also plays a role with pyrimidines > purines, and guanine derivatives > adenine derivatives. These results suggest an interaction between the 2-position substituent of the heterocyclic rings and the mineral surface, including possible interactions with surface steps, in addition to interactions between the sugar and phosphate moieties.
The above figures show the calculated proportions of the two glutamate surface species as functions of pH at two different ionic strengths and glutamate loadings.
ATR-FTIR spectra (left) of aqueous glutamate (top spectrum) and glutamate adsorbed to rutile surfaces indicate two glutamate surface species (A); calculation of glutamate surface complex in (right) attached to a cluster from the (110) surface of rutile with hydrogen-bonded water molecules. Atoms of Ti, C, N, O and H are colored, beige, grey, blue-grey, green, and red, respectively.
Publications
-
(no authors found) (2010). Workshop OQOL’09: Open Questions on the Origins of Life 2009. Orig Life Evol Biosph, 40(4-5), 347–497. doi:10.1007/s11084-010-9213-2
-
Cleaves II, H. J. (2010). The origin of the biologically coded amino acids. Journal of Theoretical Biology, 263(4), 490–498. doi:10.1016/j.jtbi.2009.12.014
-
Cleaves, H. J., Jonsson, C. M., Jonsson, C. L., Sverjensky, D. A., & Hazen, R. M. (2010). Adsorption of Nucleic Acid Components on Rutile (TiO 2 ) Surfaces. Astrobiology, 10(3), 311–323. doi:10.1089/ast.2009.0397
-
Jonsson, C. M., Jonsson, C. L., Estrada, C., Sverjensky, D. A., Cleaves, H. J., & Hazen, R. M. (2010). Adsorption of l-aspartate to rutile (α-TiO2): Experimental and theoretical surface complexation studies. Geochimica et Cosmochimica Acta, 74(8), 2356–2367. doi:10.1016/j.gca.2010.01.003
-
Jonsson, C. M., Jonsson, C. L., Sverjensky, D. A., Cleaves, H. J., & Hazen, R. M. (2009). Attachment of l -Glutamate to Rutile (α-TiO 2 ): A Potentiometric, Adsorption, and Surface Complexation Study. Langmuir, 25(20), 12127–12135. doi:10.1021/la901635t
- Cleaves, H.J. & Bada, J.L. (in press). The prebiotic chemistry of alternative nucleic acids. In: Gordon, J.S.a.R. (Eds.). Genesis: Origin of Life on Earth and Planets. Berlin: Springer.
- Cleaves, H.J. (2010). The prebiotic synthesis of RNA and pre-RNA. Origins of Life Evol. Biosphere, 40: 433.
- Marshall-Bowman, K., Sverjensky, D.A., Hazen, R.M. & Cleaves, H.J. (2010). Mineral catalysis of peptide bond cleavage. Geochim. Cosmochim. Acta, 74: 5852-5861.
- Ohara, S. & Cody, G.D. (2010). Surface-catalyzed peptide formation on sulfide minerals. Astrobiology Science Conference 2010, LPI Contribution 1538: 5309.
- Peters, J.W.C.G., Russell, M., Ferry, J.G. & Schoonen, M.A.A. (2010). Prebiotic Organometallic Catalysis. Astrobiology Science Conference 2010, LPI Contribution 1538: 5566.
- Sherwood-Lollar, B., Morrill, P.L., Cody, G.D., Fogel, M.L., Lacrampe-Couloume, G., McCollum, T.M., Seewald, J.S. & Weinberger, D. (2010). Bridging the gap between experimental and field investigations of abiotic hydrocarbon synthesis. Astrobiology Science Conference 2010, LPI Contribution 1583: 5279.
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Chrisopher Johnson
Collaborator
Jennifer Stern
Collaborator
Caroline Jonsson
Postdoc
Shohei Ohara
Postdoc
Nabil Boctor
Research Staff
Henderson Cleaves
Research Staff
Rachel Maxwell
Undergraduate Student
-
RELATED OBJECTIVES:
Objective 3.1
Sources of prebiotic materials and catalysts
Objective 3.2
Origins and evolution of functional biomolecules