2007 Annual Science Report
 Carnegie Institution of Washington
Reporting | JUL 2006 – JUN 2007
Carnegie Institution of Washington
Reporting | JUL 2006 – JUN 2007
Project 3. Prebiotic Chemical and Isotopic Evolution on Earth
Project Summary
Project Progress
1. Earth’s Early Sulfur Cycle
University of Maryland Doctoral Student David Johnston and Co-Investigator James Farquhar determined relationships among the four isotopes of sulfur to provide key evidence that a longstanding model for dissimilatory sulfate reduction proposed by Rees in 1973 needs to be revised and that a recently proposed model by Benjamin Brunner and Stephano Bernasconi in 2005 can explain the data. This type of data was lacking at the time of the Brunner and Bernasconi proposal. Johnston also used the same techniques to document evidence for a temperature dependence to the internal fractionations for sulfate reduction. Postdoctoral Fellow Aubrey Zerkle, who is working with Farquhar, undertook an investigation of phototrophic sulfide oxidation and found that the fractionations produced by phototrophic sulfide oxidation can be explained in terms of metabolic models of the same type as those proposed for dissimilatory sulfate reduction. Other work by Farquhar included a study of Astrobiology Drilling Program Core 9 using a combined sulfur hexafluoride and SO technique. This study provided high-resolution data for the drill core that exhibits clear correlations with other drill cores from the same basin studied by former Postdoctoral Fellow Shuhei Ono (now at MIT). The features of these data also point to clear changes in the sulfur isotope systematics that can be correlated as a function of stratigraphic position within the core and will be a focus for future studies using microanalytical techniques.
Co-Investigator Douglas Rumble, with collaborators Minik Rosing (Copenhagen), Samuel Bowring (MIT), Yuichiro Ueno and Tsuyoshi Komiya (both Tokyo Institute of Technology), investigated the transition from Hadean to Archean Earth at 4 Ga by analyzing a variety of rocks and minerals for 17O/16O in addition to the more commonly analyzed 18O/16O. The data base of analyzed Hadean, Archean, and Proterozoic samples includes localities at Acasta (4.1 Ga), Isua (3.8 Ga), Barberton Mountain Land (3.2 Ga), and the Neoproterozoic of central China (0.75 Ga) as well as recent samples reported in the literature. No trace has been found of the 17O anomalies that might have been left behind by meteorites hitting Earth during the episode of late heavy bombardment that so dramatically altered the face of the Moon. The 16O-17O-18O systematics of Earth are uniform throughout the past 4.1 Gy. The results place a strong constraint on the minimum age, 4.1 Ga, of the events that exchanged and equilibrated Earth’s oxygen throughout the crust and mantle. These events include accretion, global melting, planetary differentiation and core separation, and the catastrophic exclamation point of the Moon-forming giant impact. The oxygen stable isotope data are consistent with studies of the daughter products of short-lived radionuclides that demonstrate that the construction of Hadean Earth was completed within the first 100 Ma following accretion from the solar nebula.
Rumble and collaborators Huiming Bao (Louisiana State University) and Donald Lowe (Stanford University) initiated an intensive investigation of Archean barite (BaSO4) samples from the 3.2-Ga Barberton Mountain Land. They have analyzed 16O-17O-18O and 32S-33S-34S-36S in aliquots of the same barite samples. To date, the group’s analytical results show neither correlation between δ18O and Δ33S nor between δ34S and Δ33S. Two working hypotheses are being considered to explain the data: (a) An overwhelming majority of the sulfate in the Archean ocean originated by photolysis, or (b) the early Archean sulfate was a mixture of 33S-normal sulfates at the surface with a small portion of photolysis-sulfate produced from the atmosphere.
Ono was awarded, together with Nicolas Beukes (University of Johannesburg) and Co-Investigators Marilyn Fogel and Rumble, the Jubilee Prize of the South African Geological Society for the best article published in their journal for 2006. Ono and co-authors reported on sulfur and carbon isotope analyses of 2.9-Ga rocks of the Mozaan Group, South Africa, and their relationship to evolution of the atmosphere.
2. The Critical Role of Sulfur in Prebiotic (Protometabolic) Organic Chemistry
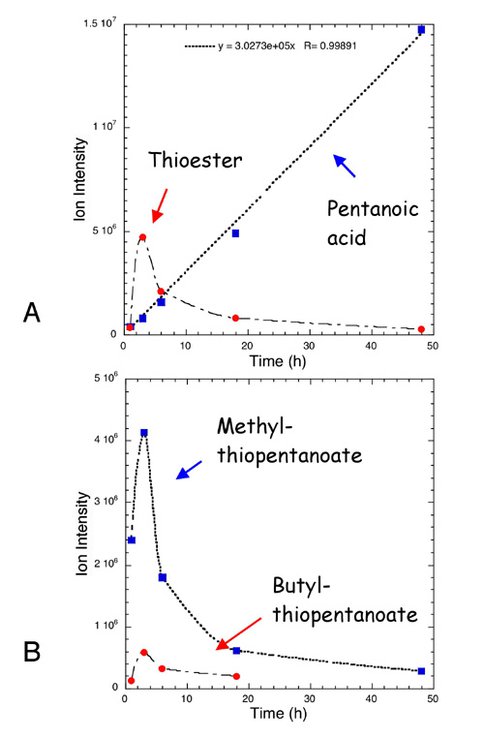
Co-Investigator George Cody and Collaborator Nabil Boctor continued their experimental studies of metal-sulfide-promoted prebiotic chemistry. Previous work outlined a potential entry point into a chemical reaction network that includes many compounds familiar to intermediary metabolism, e.g., citric acid and pyruvic acid. Over the past year Cody, Boctor, and NAI Postdoctoral Fellow Jennifer Stern (NASA Ames Research Center NAI team) focused on establishing the magnitude of kinetic isotope fractionation associated with carbon fixation via hydrocarboxylation, a metal-sulfide-catalyzed reaction that mimics the action of acetyl CoA synthase. In order to maximize the isotopic signal we used butane thiol as the addition subtrate. Butane thiol is barely a liquid at room temperature and very difficult to work within the weld stage of the sample preparation. After several false starts using our standard approach we developed a new sample loading method that allows us to explore a much wider range of small molecule reactions. An example of the quality of data we can now achieve is shown in Figure 1.
The results presented in Figure 1 verify that the reactions are surface catalyzed. Furthermore, the very rapid formation of both methyl and butyl thioesters requires a surface-catalyzed reaction, due to the relatively high yield with a low background concentration of pentanoic acid. These developments are significant and provide considerable credibility to the idea that transition-metal sulfides could have provided important catalytic functionality to the early Earth.
Over the past year Cody and Boctor have continued their efforts to synthesize a new suite of minerals to explore their catalytic qualities. In particular they synthesized tungstenite (WS2), molybdenite (MoS2), and awaruite (Ni3Fe). These minerals are found in aqueously altered ultramafic rocks. Preliminary results reveal substantial catalytic activity for carbonyl insertion chemistry. The team is in the process of obtaining stable isotopic data in order to refine their ability to distinguish between abiotic and biotic synthesized hydrocarbons.
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
James Farquhar
Co-Investigator
Douglas Rumble
Co-Investigator
Huiming Bao
Collaborator
Nabil Boctor
Collaborator
Samuel Bowring
Collaborator
Jay Brandes
Collaborator
Mario Fiorentini
Collaborator
Tsuyoshi Komiya
Collaborator
Donald Lowe
Collaborator
Shuhei Ono
Collaborator
Minik T. Rosing
Collaborator
Jennifer Stern
Collaborator
Yuichiro Ueno
Collaborator
Andrey Bekker
Postdoc
Boswell Wing
Postdoc
Aubrey Zerkle
Postdoc
David Johnston
Doctoral Student
Margaret Baker
Graduate Student
-
RELATED OBJECTIVES:
Objective 3.1
Sources of prebiotic materials and catalysts
Objective 4.1
Earth's early biosphere
Objective 4.2
Foundations of complex life
Objective 7.1
Biosignatures to be sought in Solar System materials