2004 Annual Science Report
 Carnegie Institution of Washington
Reporting | JUL 2003 – JUN 2004
Carnegie Institution of Washington
Reporting | JUL 2003 – JUN 2004
Prebiotic Molecular Selection and Organization
Project Summary
Life emerged on Earth as a geochemical process, the consequence of ancient interactions among rocks, water, and gases.
Project Progress
Life emerged on Earth as a geochemical process, the consequence of ancient interactions among rocks, water, and gases. More than a half-century of research has demonstrated the facile synthesis of biomolecules in many prebiotic environments, from deep space to Earth’s deep interior. However, the processes by which that diverse prebiotic collection of molecules were selected, concentrated, and organized into the essential macromolecules of life constitute a significant gap in our understanding of life’s origins. During the past year our studies of prebiotic molecular selection and organization have focused on two aspects of this history: (1) molecular self-organization, and (2) molecular selection and organization on mineral surfaces.
Molecular Self-Organization
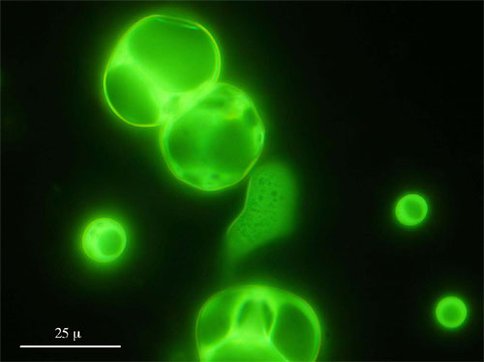
Lipids. Deamer, Hazen, and colleagues continued their studies of amphiphile self-organization with investigations of the self-assembly of molecules produced when an aqueous solution of pyruvate is subjected to high-pressure (to 400 MPa) and temperature (to 300°C). The group observed facile amphiphile synthesis and subsequent self-assembly of products that mimic those from carbonaceous chondrites and vacuum-ultraviolet (UV) experiments (Figure 1).
The “PAH World.” In a related effort, Carnegie Predoctoral Fellow Nicholas Platts (Renssalaer Polytechnic Institute) proposed a novel hypothesis, dubbed the “PAH world,” related to prebiotic molecular selection and organization. He suggested that the chemical behavior of polycyclic aromatic hydrocarbons (PAHs) provides a plausible solution to a central problem in origin-of-life science: the link between the dilute prebiotic mixture of small molecules on the one hand, and the formation of molecules with capacity to carry genetic information on the other. In his proposal the dilute “prebiotic soup” contained abundant PAHs, which could have spontaneously self-assembled into stacked, or discotic, arrangements. Under this scenario, PAH molecules of roughly similar size and shape stack one above the other, thus bringing their edges into approximate alignment. Lateral and rotational relative motions of individual stacked PAH molecules would effectively select a random distribution of small, flat, edge-bound molecules (i.e., the nucleotide bases) from the random aqueous environment (Figure 2). This proposed “PAH world” logically precedes the “RNA world” for the origin of life. The model makes a sequence of testable predictions related to the self-assembly of derivatized PAHs, the chemical selectivity for edge-bound bases, and the subsequent oligomerization of these units. Experiments to test these predictions are underway.
{{ 2 }}
Figure 2. This diagrammatic figure conveys the essential features of the “PAH world” hypothesis of Platts and others. Three prebiotic nucleobases are shown hydrogen bonded to hydroxy functions in the edge structures of some derivatized and neighboring PAH molecules, which are themselves disposed in a stacked and discotic array. The exemplar polynuclear aromatic compound being envisaged here is 1,3-dihydroxy hexa- peri -hexabenzocoronene (C42H18O2). The hydroxy functionalities are merely meant to be illustrative of the general chemical principle of derivatization at the edges of PAH molecules; other types of organic functionalization/derivatization (e.g., -COOH, C=O) are certainly possible, especially given the typical productive richness of organic photochemistry. The PAHs shown here are partially hydroxylated, and are thus also partially oxidized relative to the parent aromatic hydrocarbons. The interplanar (i.e., inter-PAH) spacings would be expected to be approximately 0.34 nm (cf. 0.335 nm in graphite, and the 0.34-nm inter-residue helical rise in the B crystallographic form of DNA). The three nucleobases are being shown in their keto tautomeric forms (i.e., with protonated ring nitrogens), so as to be consistent with the seemingly reasonable assumption that prebiotic Hadean ( i.e., > ca. 3.85 Ga) surface and rain waters were probably acidulous [e.g., an otherwise inert Hadean atmosphere with a PCO2(g) of 0.10 bar (i.e., ~271.0 PAL CO2(g) ) would equate to pH ~4.4 (298.15 K) for rain/surface waters]. The keto tautomers bear the pro-glycosidic purine and pyrimidine secondary amine nitrogens (i.e., the NH functions at N(9) and N(1), respectively) disposed outwards, and thus chemically accessible, to the surrounding solution. The derivatized PAH edge structures effectively represent something of an electronic phase boundary between the discotically arrayed aromatic cores of the PAHs and the surrounding aqueous media of Darwin ‘s “warm little pond.” The phenomenon of life can thus be seen as deriving from what is effectively a phase separation between the discotic aromatic cores of the PAHs versus the surrounding aqueous based phase. It seems probable to us that the essentially regular (2pz) inter-base plane-parallel spacing distance of 0.34 nm was inherited by molecular biology as a chemical memory of this unique mode of origin, and that this structural molecular fossil has been conserved in the essentially semi-discotic dispositions of the heterocyclic bases in the secondary and higher order structures of extant nucleic acids.Molecular Selection and Organization on Mineral Surfaces
Selection of Amino Acids. Of special interest are the mechanisms by which molecules are selectively adsorbed from an aqueous solution. Hazen and colleagues characterized the surfaces of calcite, quartz, and feldspar using atomic force microscopy and demonstrated that maximum adsorption of amino acids occurs when the mineral’s point of zero charge and the amino acid’s isoelectric point differ significantly. They also initiated a project to model molecule-crystal interactions from first principles with density functional theory.
Chiral Selection. One of life’s most striking biochemical characteristics is its preference for chiral, or handed, molecules. All known cells use predominantly left-handed amino acids and right-handed sugars, for example. However, all prebiotic synthesis pathways yield equal amounts of left- and right-handed molecules. A key step in understanding the origin of life, therefore, is how the chiral selection process occurred.
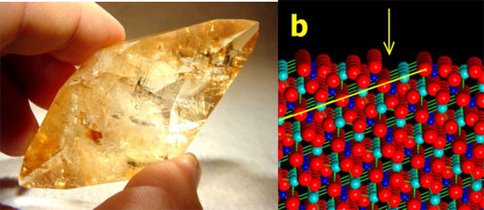
Hazen, Asthagiri, Maule, and Steele continued investigations of the possible roles of chiral mineral surfaces, which are ubiquitous in nature. They began a systematic characterization of the most common chiral surfaces of rock-forming minerals, including quartz, calcite, feldspar, pyroxene, and amphibole (Figure 3). They found that surfaces featuring both positive and negative charge centers, including calcite, pyroxenes, and feldspars, have a greater intrinsic ability to adsorb selectively left- and right-handed molecules from solution.
In an important new direction, they applied DNA ChipWriter/ChipReader technology to study the adsorption of chiral molecules on a variety of mineral surfaces. They decorated mineral surfaces with small droplets of various solutions. Initial results demonstrate strongly selective adsorption of some chiral amino acids onto quartz and calcite crystal surfaces.
These results point to a viable mechanism for the prebiotic selection, concentration, and ordering of chiral molecules. The new data, furthermore, are proving of great interest to the pharmaceutical industry. The often-difficult chiral separation of molecules costs consumers billions of dollars annually, and mineral surfaces point to new strategies for the safe and efficient chiral purification of drugs.
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Aravind Asthagiri
Postdoc
Jake Maule
Postdoc
Simon Platts
Doctoral Student
-
RELATED OBJECTIVES:
Objective 3.1
Sources of prebiotic materials and catalysts
Objective 3.2
Origins and evolution of functional biomolecules
Objective 3.4
Origins of cellularity and protobiological systems
Objective 7.1
Biosignatures to be sought in Solar System materials