2003 Annual Science Report
 Carnegie Institution of Washington
Reporting | JUL 2002 – JUN 2003
Carnegie Institution of Washington
Reporting | JUL 2002 – JUN 2003
Hydrothermal Organic Synthesis
Project Summary
Over the past year Cody and colleagues have continued their research into hydrothermal protometabolic chemistry.
Project Progress
Task 1. Hydrothermal Organic Chemistry (Cody, Boctor, Brandes, Hazen, Yoder)
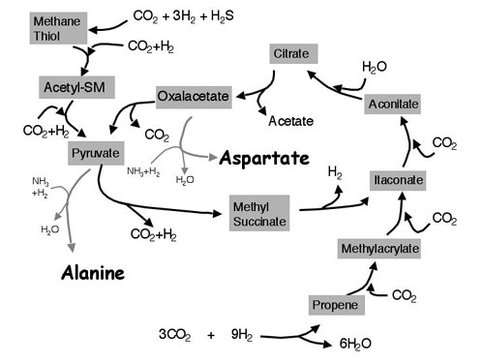
Over the past year Cody and colleagues have continued their research into hydrothermal protometabolic chemistry. Building on their previous studies, they have now demonstrated a viable pathway for the formation of alanine and aspartic acid (Figure 1). They have shown that the presence of transition-metal sulfides enhances these reactions, presumably by operating as a hydrogenation catalyst to enhance the reductive amination reaction. The group also carried out experiments coupling active nitrogen into the previously defined protometabolic carbon-fixation pathways with the aim of establishing evidence of pathways that may lead to pyrimidine synthesis, one threshold to the “RNA-world.”
Hazen and Brandes also performed a number of prebiotic synthesis experiments involving simple molecules in hydrothermal environments, including measurements of the conversion of nitrate and nitrite to ammonia at elevated pressures (to 0.2 GPa) and temperatures (to 250°C)
Task 2. Amino Acid Synthesis Under Hydrothermal Conditions (Bischoff, Ross, Lemke)
The U.S. Geological Service (USGS) group’s activities include study of oligomerization and decarboxylation of amino acids in hydrothermal media, as well as modeling the fate of amino acids arriving in impacting cometary bodies. They showed that, while in accord with literature accounts, the formation of glycine dipeptide in hydrothermal media is endergonic by 2-4 kJ/mole, and the next step to the tripeptide is exergonic by 10-12 kJ/mole. This value is presumably applicable to all subsequent growth steps. The downhill swing of up to 16 kJ/mole provides an answer to the long-standing question of the spontaneous formation of polypeptides on the early Earth, and kinetics estimates at 25°C suggest that considerable levels of oligomerization would be expected over periods of a few millions of years in local regions of high concentrations. Since life probably began within a 100-million-year span following the end of heavy bombardment, an open ocean scenario with amino acid concentrations below the mM range is not possible, inasmuch as kinetics estimates show the entire period would be required for just the first step to the dipeptide.
This obstacle has led to an exogenous alternative that provides a surprisingly facile course to functional, chiral polypeptides utilizing polyamino acids derived directly from incoming comets. The group’s present estimates, developed in collaboration with Elizabetta Pierazzo at the University of Arizona, suggest that for some impacts the amino acids in the collider survive the event and then quickly form polymers during cooling. Beginning with this “jump start” to biochemistry the group’s analysis employs statistical estimates of random chiral segments in the racemic polymers. Kinetics estimates for the hydrolysis of achiral and homochiral peptide segments then reveal the hydrolytic stability of the latter, which in turn can provide chiral, self-replicating and autocatalytically functional polypeptides within 40 to 50 million years.
Task 3. Stability of Organic Compounds at Very High Pressure (Hemley, H. Scott)
Over the past year Hemley and NAI postdoctoral fellow Henry Scott have used diamond anvil cells (DACs) to make in situ observations of the oxidation and reduction of carbon at simultaneously high pressures and temperatures. It is likely that planetary bodies accrete large amounts of reduced carbon, but it is not known how well organic species withstand hydrothermal processing at the elevated pressures of planetary interiors. Hemley and Scott conducted studies on the stability of polyethylene at a range of pressures (up to 10 GPa), temperatures, and oxygen environments. With the addition of CaSiO3 (wollastonite) to the system of polyethylene plus water, production of carbonate at the expense of the organic species is observed. Also frequently documented is a reversal reaction in the system FeO + CaCO3 + water by producing methane at 5 GPa pressure and temperatures greater than 1800 K.
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Harold Morowitz
Co-Investigator
Nabil Boctor
Collaborator
Jay Brandes
Collaborator
David Ross
Collaborator
Henry Scott
Postdoctoral Fellow
Kono Lemke
Graduate Student
-
RELATED OBJECTIVES:
Objective 3.1
Sources of prebiotic materials and catalysts
Objective 3.2
Origins and evolution of functional biomolecules
Objective 4.2
Foundations of complex life