2015 Annual Science Report
 University of Wisconsin
Reporting | JAN 2015 – DEC 2015
University of Wisconsin
Reporting | JAN 2015 – DEC 2015
Project 4A: New in Situ Techniques (CLSM and Raman) Solve the Problem Presented by the Disaggregation of Acid-Macerated Organic-Walled Microfossils
Project Summary
Because of the enormous costs involved in Mars Surface Sample Return Missions, the search for evidence of past life in rocks from Mars is likely to hinge on the use of “safe,” non-intrusive, non-destructive techniques to establish the biogenicity of any detected fossil-like objects by analyses of their cellular morphology and molecular composition. The most promising rock types to preserve such evidence are chemically precipitated sediments such as cherts, gypsums, carbonates and phosphates — all of which on Earth have been shown to be richly fossiliferous. The organic-walled microbes in such rocks are typically not amenable to investigation by the commonly used but rock-destroying technique of acid maceration. This study shows that the combined use of optical microscopy, confocal laser scanning microscopy, and Raman spectroscopy solves this problem, documenting effective means for the investigation of Mars rocks.
Project Progress
Although the most life-like, “best preserved” fossils in the geological record are those permineralized (“petrified”) in quartz, calcite, apatite or gypsum, the vast majority (>99%) of organic-walled microfossils (e.g., bacteria, fungi, microalgae, acritarchs, spores and pollen) are preserved as compressions, two-dimensional flattened remains compressed between layers of siltstone or shale. Studies of such compression-preserved fossils in situ are far less informative than such studies of three dimensionally preserved permineralized fossils.
Nevertheless, compression-preserved microfossils are widespread and especially well known, studied intensively by micropaleontologists worldwide. To identify such specimens and elucidate their morphology, most workers dissolve the fossiliferous rocks in mineral acids (e.g., HF, HCl) and study the macerated acid-resistant organic residues by use of optical or scanning electron microscopy. Although appropriate for compression-preserved fossils, this technique does not work well for permineralized fossils, the cell walls of which are mineral-infused, supported by the embedding mineral matrix which as it dissolves during acid maceration commonly results in their disaggregation.
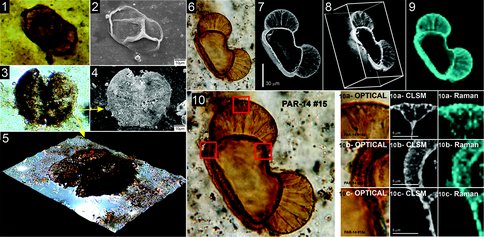
Comparison of results obtained from studies of permineralized ~278 Ma pine pollen in acid-macerates and embedded within the rock matrix illustrates both the problem and its solution. Freed from their embedding matrix, the permineralized acid macerated pollen grains collapse, flatten and provide minimal useful information. In contrast, much useful information at high spatial resolution can be obtained in situ by the use of three non-intrusive, non-destructive techniques: optical microscopy, confocal laser scanning microscopy (CLSM), and Raman.
As is shown in Figures 1-10 (Schopf et al., Submitted), the combined use of these three techniques has numerous advantages:
(1) Unlike the two-dimensional images provided by standard optical photomicrography, structural information provided by CLSM images is three-dimensional and can be rotated and viewed from any desired perspective.
(2) Unlike SEM, which for acid-macerated fossils provides topographic images of the exterior surface of flattened specimens, CLSM images of specimens that transect the thin section surface can provide fine-structural detail of the interior structure of cell walls — lateral spatial resolution of ~200 nm, 50% higher than standard optical microscopy.
(3) Because CLSM detects laser-induced fluorescence emanating from the polycyclic aromatic hydrocarbons that dominate the carbonaceous kerogen of fossil cell walls, CLSM serves as a proxy for analyses of chemical composition.
(4) Raman spectroscopy provides spectral data that establish the chemical composition of the embedding mineral matrix as well as that of fossil kerogenous cell walls.
Publications
-
Schopf, J. W., CalçA, C. P., Garcia, A. K., Kudryavtsev, A. B., Souza, P. A., Félix, C. M., & Fairchild, T. R. (2016). In situ confocal laser scanning microscopy and Raman spectroscopy of bisaccate pollen from the Irati Subgroup (Permian, Paraná Basin, Brazil): Comparison with acid-macerated specimens. Review of Palaeobotany and Palynology, 233, 169–175. doi:10.1016/j.revpalbo.2016.03.004
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Ciéber Caiça
Collaborator
Thomas Fairchild
Collaborator
Amanda Garcia
Collaborator
Anatoliy Kudryavtsev
Collaborator
-
RELATED OBJECTIVES:
Objective 7.2
Biosignatures to be sought in nearby planetary systems