2015 Annual Science Report
 NASA Jet Propulsion Laboratory - Icy Worlds
Reporting | JAN 2015 – DEC 2015
NASA Jet Propulsion Laboratory - Icy Worlds
Reporting | JAN 2015 – DEC 2015
Inv 2 – From Geochemistry to Biochemistry
Project Summary
INV 2 focuses on experimentally simulating the geological disequilibrium in hydrothermal systems, and determining the role of minerals in harnessing these gradients toward the emergence of metabolism. Biology utilizes metals (to speed up reactions) and “engines” (such as electron bifurcators, to couple endergonic and exergonic reactions); these components in modern metabolism strongly resemble specific minerals found in hydrothermal environments. We focus on simulating these primordial geological components and processes that might have led to the beginning of metabolism in a seafloor system on a wet rocky planet.
Project Progress
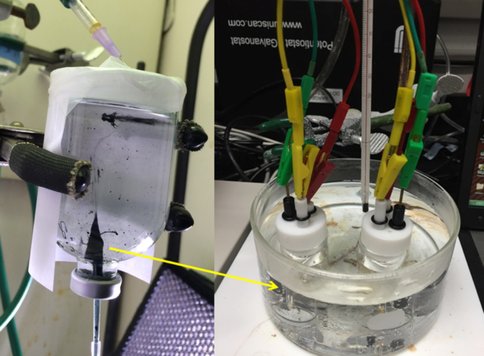
To simulate the far-from-equilibrium system of alkaline vents, new experimental techniques have been developed – namely, “Protometabolic Electrochemical Reactor” (PEM) experiments, which directly interface simulated ocean and hydrothermal solutions to produce catalytic chimney material and allow measurement of the proton/electron/ion gradients across the chimney wall. Our initial experiments demonstrated that simulated prebiotic hydrothermal chimneys generate electrical potential and current (as chimneys do in modern field settings; Yamamoto et al. 2014), and that the particulars of ocean/vent fluid composition (e.g. concentration of reductants such as sulfide or oxidants such as nitrate/nitrite) greatly affect the gradients generated in the system (Barge et al. 2015a). We also developed methods to vary the geochemical gradients by ‘switching’ hydrothermal fluid compositions during chimney growth, as may happen in a natural setting as the geochemistry evolves. Our results showed that, although the gradients across a chimney are a result of the solution chemistry, the ability of the chimney itself to act as a redox catalyst may depend on past hydrothermal chemistry that led to precipitation of particular minerals (Barge et al. 2015b).
Based on this work, PEM experiments were conducted in summer 2015 by JPL undergraduate interns T. Lin and R. Cameron, in order to test whether electric potentials across chimney walls can drive carbon dioxide reduction. Major takeaways from this work were 1) the successful synthesis of hydrothermal catalysts and applying these catalysts onto carbon electrodes, for use in 3-electrode half-cell experiments that simulate the redox chemistry on either side of the chimney wall; and 2) developing techniques for conducting the experiments at higher temperature which will allow simulation of the hydrothermal chimney interior.
We are currently working with USC NAI collaborators (Collaborator Nealson) on electrode fabrication methods for these experiments, so that we can grow minerals in and on the electrodes, in order to most accurately simulate the generation of electrical potential and current in hydrothermal chimneys.
These initial method development efforts now allow for laboratory simulation of any particular hydrothermal system on early Earth or an icy world, thus preparing us to incorporate results from our other Investigations regarding icy world ocean / vent fluid composition. From our previous work (White et al. 2015), we learned that, at 70 degrees Celsius, lab-grown hydrothermal chimneys incorporate the catalytic iron sulfide mineral greigite, which is a “proto-metalloenzyme” catalyst for CO2 reduction (Yamaguchi et al. 2015). Recent exciting work by Wang et al. (2015) has shown that, in the presence of simple organic acids that would be expected in vent systems (e.g. pyruvate), greigite can form even at room temperature. This prompted us to develop an additional research focus on testing the effects of organic/inorganic catalytic diversity in metal sulfide chimney systems, in particular to induce organic dopant/carbon reduction feedbacks to understand the early evolution of enzymes. This project was recently funded as a NAI DDF award in Jan 2016, including substantial collaboration with the NAI SETI team and the Earth-Life Science Institute at Tokyo Institute of Technology.
In addition to far-from-equilibrium systems experiments, we have also made progress on testing specific hydrothermal mineral catalysts that are proposed to play important roles in the emergence of metabolism. One mineral of great interest is “green rust” (GR), a double-layer, mixed valence iron oxyhydroxide mineral that is known for its ability to intercalate anionic species (including organics) and also catalyze redox reactions. Thus far, the gap in understanding GR chemistry has been the controlled, strictly anaerobic synthesis of particular GR types for evaluation of their reactivity. Thus the synthesis of GR has been a focus for the collaboration between JPL and the Oak Crest Institute of Science. Initial results are promising: Oak Crest undergraduate researcher V. Aguirre has synthesized a range of GR samples with rigorously controlled pH and redox potentials under strict anaerobic conditions, leading to well-defined Fe2+/Fe3+ oxidation ratios and avoiding the formation of undesirable oxidation or by-products common to other methods. Meanwhile, JPL student interns have also developed methods to synthesize “Early Earth” GR representing early Earth seafloor / hydrothermal precipitates, formed by mixing of simulated Hadean ocean water and alkaline hydrothermal vent fluid.

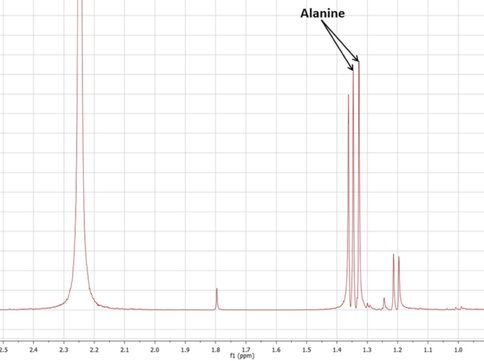
Thus far, we have found that the various forms of GR possess significant differences in stability and reactivity with regards to driving proto-metabolic reactions. For one thing, our results show that under many conditions, GR is able to generate ammonia from “oceanic” nitrate – an eight electron reduction! This simple mechanism of ammonia generation in GR-containing vent systems prompted us to begin testing the formation of amino acids via the reductive amination of pyruvate (e.g. Novikov and Copley 2013, Huber and Wächtershäuser 2003). This reaction has previously been shown to occur in the presence of metal sulfide or ferrous hydroxide catalysts, but the presence of mixed-valence highly reactive GR minerals had never been experimentally tested. In prebiotic hydrothermal systems, minerals do not only catalyze reactions, but they direct the pathways that reactions are likely to take – and we have so far found that, in systems containing “early Earth seafloor” GR, alanine is synthesized from pyruvate and ammonia in 24-48 hours.
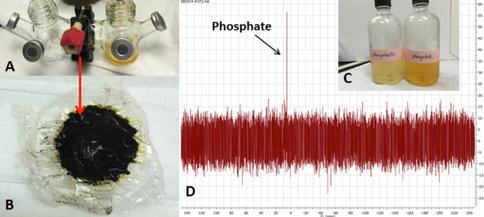
This project is the ongoing work of E. Flores, a former JPL intern with our team and currently a JPL Engineer Tech; her future experiments will include evaluating alanine synthesis, concentration, and possibly polymerization in simulated GR- and sulfide- containing chimneys. GR is also well-known for its ability to concentrate phosphate, and JPL intern Y. Abedian has begun testing the ability of GR-containing chimneys to absorb and concentrate phosphorus species in the early oceans. Her preliminary results have shown that, even though phosphorus may only be present in very dilute amounts in the oceans, a GR-containing hydrothermal precipitate or chimney can concentrate and retain phosphate/phosphite to levels relevant for prebiotic reactions.
These laboratory experiments, though preliminary, are painting a picture of the complex network of reactions that might have occurred in a hydrothermal mound on the early Earth or on an icy world – combining geological gradients and redox reactions, with organic synthesis and concentration, and linking both of these to the generation of energy currency. Taken together, these experiments are advancing us toward being able to experimentally test, for the first time, the detailed theoretical work being carried out by Russell and collaborators from the NAI-UIUC team regarding the possible ability of GR to promote methane oxidation (with NOx reduction) and the synthesis of phosphate energy currency at the origin of life (Russell et al. 2014).
References:
Barge L. M. et al. (2015a) Angew. Chem. Int. Ed., 54, 28:8184-8187, DOI: 10.1002/anie.201501663.
Barge L.M. et al. (2015b) JoVE, doi: 10.3791/53015.
Huber C. and Wächtershäuser G. 2003, Tetrahedron Letters 44: 1695–1697.
Novikov Y. and Copley S.D. (2013) PNAS 110, 33:13283–13288.
Russell, M. J., et al. (2014) Astrobiology 14, 4, 308-343.
Wang, W. et al. 2015, Astroiology 15, 12, DOI: 10.1089/ast.2015.1373.
White L.M. et al. 2015, EPSL 430: 105-114.
Yamaguchi A. et al. 2015, Electrochim. Acta, doi:10.1016/j.electacta.2014.07.078 4.
Publications
-
Barge, L. M., Abedian, Y., Doloboff, I. J., Nuñez, J. E., Russell, M. J., Kidd, R. D., & Kanik, I. (2015). Chemical Gardens as Flow-through Reactors Simulating Natural Hydrothermal Systems. JoVE, None(105), None. doi:10.3791/53015
-
Barge, L. M., Abedian, Y., Russell, M. J., Doloboff, I. J., Cartwright, J. H. E., Kidd, R. D., & Kanik, I. (2015). From Chemical Gardens to Fuel Cells: Generation of Electrical Potential and Current Across Self-Assembling Iron Mineral Membranes. Angew. Chem. Int. Ed., 54(28), 8184–8187. doi:10.1002/anie.201501663
-
Barge, L. M., Cardoso, S. S. S., Cartwright, J. H. E., Cooper, G. J. T., Cronin, L., De Wit, A., … Thomas, N. L. (2015). From Chemical Gardens to Chemobrionics. Chem. Rev., 115(16), 8652–8703. doi:10.1021/acs.chemrev.5b00014
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Marc Baum
Co-Investigator
Michael Russell
Co-Investigator
Yeghegis Abedian
Collaborator
Vincent Aguirre
Collaborator
Ryan Cameron
Collaborator
Scott Churchman
Collaborator
Erika Flores
Collaborator
John Moss
Collaborator
Kenneth Nealson
Collaborator
David VanderVelde
Collaborator
-
RELATED OBJECTIVES:
Objective 3.1
Sources of prebiotic materials and catalysts
Objective 3.2
Origins and evolution of functional biomolecules
Objective 3.3
Origins of energy transduction
Objective 4.1
Earth's early biosphere.
Objective 7.1
Biosignatures to be sought in Solar System materials