2015 Annual Science Report
 NASA Ames Research Center
Reporting | JAN 2015 – DEC 2015
NASA Ames Research Center
Reporting | JAN 2015 – DEC 2015
Laboratory Studies
Project Summary
The Laboratory Studies project uses a variety of cryo-vacuum systems to study the physical and chemical properties of astrophysically relevant materials to better understand the extent to which these materials can be converted in more complex organic materials of astrophysical and astrobiological importance. We concentrate on mimicking conditions found in astrophysically relevant environments involving low temperatures, low pressures, and high radiation fields. The main processes we explore are the photolytic processing of mixed molecular ices and organics and chemistry that occurs at gas-solid interfaces.
Project Progress
During the past year our laboratory efforts have concentrated on the study of the catalytic chemistry that occurs at gas-solid interfaces and the production of organic molecules of astrobiological interest that occurs when mixed molecular ices are exposed to ionizing radiation. Specific classes of compounds we investigated in 2015 include nucleobases and other heterocyclic aromatic hydrocarbons, sugars and sugar-related compounds, and organics made in simulations of Pluto’s surface. Brief summaries of each effort are provided below.
Gas-Solid Chemistry
During the first year of the grant we acquired a Harrick Praying Mantis Diffuse Reflectance apparatus and the corresponding low temperature cell (see Figure 1). This new equipment has been successfully interfaced and tested with one of our existing vacuum systems and infrared spectrometers. It will be used to study the adsorption processes of polycyclic aromatic hydrocarbons (PAHs) and ice mixtures onto mineral grain surfaces. We will also use this apparatus to determine any catalytic properties the grains may exhibit when irradiated with high energy UV photons (primarily Lyman-α). Dr. Gustavo Cruz-Diaz recently joined the team in early December 2015 to conduct the mineral grain experiments. Dr. Cruz-Diaz is an experimentalist with extensive experience with high-vacuum systems, UV photolysis of organic samples and organic spectroscopy.
The Formation of Nucleobases and Other Heterocyclic Aromatic Hydrocarbons
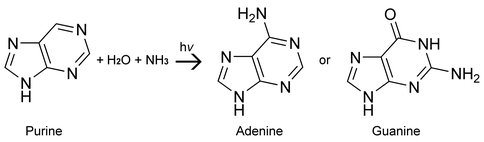
Biological nucleobases including uracil, adenine and guanine have been detected in meteorites, in addition to numerous non-biological purines and pyrimidines [1-3]. Prior work in our laboratory had focused on the formation of nucleobases based on pyrimidine under conditions designed to mimic dense molecular clouds [4-7]. These experiments involved the UV photoprocessing of pyrimidine in astrophysically relevant ices at low temperatures (~15K). This year we entered a new phase of these experiments and have begun studying the UV photoprocessing of purine in simplified astrophysical ices (e.g. H2O:NH3:purine) to see if the two purine-based nucleobases, adenine and guanine (Figure 2), are made. Preliminary results indicate that the biological nucleobase adenine is readily produced in these experiments. Guanine may also be produced, though more experiments are required to unequivocally confirm its presence. In addition to these biological nucleobases, we have also detected the presence of hypoxanthine, isoguanine, 2-aminopurine, and 2,6-diaminopurine.
[1] Hayatsu, R. et al. (1975) Geochim. Cosmochim. Acta, 39, 471.
[2] van der Velden, W. and Schwartz, A. (1977) Geochim. Cosmochim. Acta, 41, 961.
[3] Stoks, P. and Schwartz, A. (1979) Nature, 282, 709.
[4] Nuevo, M. et al. (2009) Astrobiology, 9, 683.
[5] Nuevo, M., et al. (2012) Astrobiology, 12, 295.
[6] Materese, C. K. et al. (2013) Astrobiology, 13, 948.
[7] Nuevo, M. et al. (2014) Astrophys. J., 793, 125.
The Formation of Sugars and Sugar-Related Compounds:
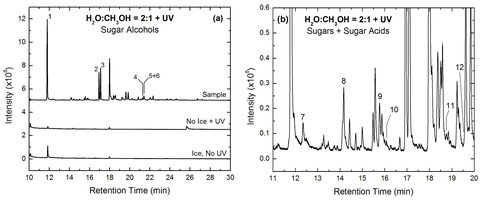
Using the same experimental techniques that are known to lead to the formation of organic molecules of prebiotic interest such as amino acids, carboxylic acids, pyrimidine-based nucleobases, amphiphiles, etc. [1–3] via the UV irradiation of ice mixtures of astrophysical relevance at low temperature (T < 15 K), we conducted a systematic search for the formation of sugars and sugar derivatives. The main motivation for this study is the detection of sugar derivatives in the Murchison and Murray carbonaceous meteorites (Figure 3) [4]. The first series of experiments consisted of the UV irradiation of simple H2O:CH3OH (2:1 and 5:1) ice mixtures. After simultaneously depositing and irradiating these ices at 15 K for 18–20 hours, we stopped the irradiation and slowly warm up the samples to room temperature. The residues covering the substrate at room temperature were analyzed using GC-MS for the search of sugars and sugar derivatives. The analysis of several of these residues showed the presence of sugar alcohols with carbon chain lengths up to 5 carbon atoms (ribitol and its isomers), as well as sugars and sugar acids containing up to 4 carbon atoms (Figure 4). However, the distribution of sugar derivatives is different from that found in meteorites, as meteoritic sugar acids were found in larger abundances, and only the smallest sugar (dihydroxyacetone) could be identified in Murchison and Murray [4]. A paper describing these results has recently been submitted for publication.
[1] Bernstein, M. P. et al. (2002) Nature, 416, 401.
[2] Nuevo, M. et al. (2008) Orig. Life Evol. Biosph., 38, 37.
[3] Nuevo, M. et al. (2014) Astrophys. J., 793, 125).
[4] Cooper, G. et al. (2001) Nature, 414, 879.
Simulations of Radiation Induced Chemistry in Simulated Pluto Ices
In 2015 we continued to carry out ice irradiation experiments exposing Pluto ice analogs to both UV photons and energetic electrons in order to better understand the organics that may be formed on the surface of Pluto. This effort demonstrated the very N-rich, highly polymerized organics are likely to be present on the surface of Pluto. This work has led to several publications [1,2] and generated laboratory spectral data that will be compared to data returned from Pluto by the New Horizons mission.
[1] Materese, C. K., et al. (2014) Astrophys. J., 813, 9 pp.
[2] Materese, C. K., et al. (2015) Astrophys. J., 813, 9 pp.
Publications
-
Cook, A. M., Ricca, A., Mattioda, A. L., Bouwman, J., Roser, J., Linnartz, H., … Allamandola, L. J. (2015). PHOTOCHEMISTRY OF POLYCYCLIC AROMATIC HYDROCARBONS IN COSMIC WATER ICE: THE ROLE OF PAH IONIZATION AND CONCENTRATION. The Astrophysical Journal, 799(1), 14. doi:10.1088/0004-637x/799/1/14
-
Materese, C. K., Cruikshank, D. P., Sandford, S. A., Imanaka, H., & Nuevo, M. (2015). ICE CHEMISTRY ON OUTER SOLAR SYSTEM BODIES: ELECTRON RADIOLYSIS OF N2-, CH4-, AND CO-CONTAINING ICES. The Astrophysical Journal, 812(2), 150. doi:10.1088/0004-637x/812/2/150
- Cottin, H., Saiagh, K., Nguyen, D., Grand, N., Benilan, Y., Cloix, M., Coll, P., Gazeau, M.C., Fray, N., Khalaf, D., Raulin, F., Stalport, F., Carrasco, N., Szopa, C., Chaput, D., Bertrand, M., Westall, F., Mattioda, A., Quinn, R., Ricco, A., Santos, O., Baratta, G.A., Strazzulla, G., Palumbo, M.E., Le Postollec, A., Dobrijevic, M., Coussot, G., Vigier, F., Vandenabeele-Trambouze, Incerti, S., and Berger, T. (2015) Photochemical studies in low Earth orbit for organic compounds related to small bodies, Titan and Mars. Current and future facilities. Bulletin de la Société Royale des Sciences de Liège, 84: 60-73.
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Andrew Mattioda
Co-Investigator
Louis Allamandola
Collaborator
Xander Tielens
Collaborator
Gustavo Cruz-Diaz
Research Staff
Christopher Materese
Research Staff
Michel Nuevo
Research Staff
-
RELATED OBJECTIVES:
Objective 3.1
Sources of prebiotic materials and catalysts
Objective 3.2
Origins and evolution of functional biomolecules
Objective 3.4
Origins of cellularity and protobiological systems

![Figure. 3. Sugars, sugar alcohols, sugar acids, and dicarboxylic sugar acids found in the Murchison and Murray meteorites [4]. We searched for the compounds in the 3 first columns. Compounds identifi](../../../../media/medialibrary/2016/02/fig3_0jAOiX2.jpg.484x0_q85.jpg)