2014 Annual Science Report
 Massachusetts Institute of Technology
Reporting | SEP 2013 – DEC 2014
Massachusetts Institute of Technology
Reporting | SEP 2013 – DEC 2014
Molecular Biosignatures of Redox-Sensitive Bacteria and Hyperthermophiles
Project Summary
The Summons lab has been researching a range of molecular and isotopic phenomena aimed at shedding light on what controls Neoproterozoic ocean redox, evolutionary trends in the abundances of molecular fossils (biomarkers) and the enigmatic natural variability carbon isotopic compositions of organic and inorganic carbon at this time. Our studies of carotenoid pigment biomarkers for green and purple sulfur bacteria have revealed that they are ubiquitous in rock extracts of Proterozoic to Paleozoic age—implying that the shallow oceans became sulfidic more frequently than previously thought. Other projects focused on the biosynthesis of another important biomaker, the hopanoids, vesicles released from marine bacteria for interaction between cells and their environment, and the molecular signatures of microbial communities in hot springs in Yellowstone National Park.
Project Progress
Ocean Redox
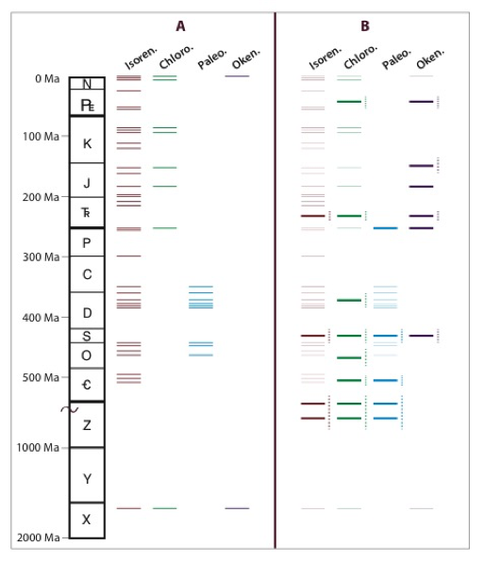
The marine biomarker record of green and purple sulfur bacteria (GSB and PSB, respectively) is a means to evaluate the fractions anoxygenic versus photosynthesis in marine primary productivity during the Precambrian as compared to the Phanerozoic. Accordingly, we developed an analysis specific to the carotenoid pigments of GSB and PSB using gas chromatography coupled to tandem mass spectrometry. Our results suggest that carotenoids derived from phototrophic sulfur bacteria, specifically okenane, chlorobactane, and paleorenieratane, are ubiquitous in marine rock extracts and oils from the Proterozoic to the Paleogene (French et al., 2015).
According to conventional paleoredox interpretations, an expanded stratigraphic distribution of the GSB and PSB biomarkers implies that the shallow sunlit surface ocean (<24 m) became sulfidic more frequently in the geologic past than was previously thought. Thus, the evidence supporting a planktonic source of GSB and PSB pigments in marine systems needs to be re-examined. To date, planktonic GSB and PSB and their pigments have been identified in restricted basins and lakes, but they have yet to be detected in the unrestricted, transiently sulfidic, marine systems. Based on modern observations, additional environmental factors, including basin restriction, microbial mats, or sediment transport are required to fully explain GSB and PSB carotenoids in the geologic record (French et al., 2015) including those identified in sediments deposited during the Toarcian oceanic anoxic event (French et al., 2014).
In a subsequent collaborative study, we examined a sequence of sediments deposited during the Triassic-Jurassic transition. Fossil molecular (biomarker) and stable N-isotopic records in sediments at Haida Gwai, western Canada that provide conclusive evidence of PZE and disrupted biogeochemistry on the northeastern Panthalassic Ocean for the end-Triassic. These results indicate the occurrence of increasing water column stratification and deoxygenation across the ETE, leading to PZE in the Early Jurassic. The development of PZE was paralleled by a perturbed nitrogen cycle, ecological turnovers among non-calcifying groups including eukaryotic algae and prokaryotic plankton. If these conditions developed widely elsewhere in the end-Triassic Panthalassic Ocean, PZE could be regarded as a potent global extinction mechanism (Kraspak et al., 2015) as it is for the end-Permian event.
Biosignature research and cross-team collaborations
The Summons lab routinely collaborates with other NASA Astrobiology members and teams to address questions related to developing and improving the robustness of biosignatures, understanding their preservation and inventing new analytical protocols.
The origin of homochirality and its role in the development of life on Earth are questions central to astrobiology. Carbonaceous chondrites, bearing amino acids with L enantiomeric excess (ee), may have seeded primitive Earth with compounds critical to life’s origin. In looking for supportive evidence, we investigated the stereochemistry of free monocarboxylic acids (MCAs) from CM2 meteorites LON 94101 and Murchison; and (2) the aliphatic side chains present in the insoluble organic matter (IOM) in the form of monocarboxylic acids (MCAs) from EET 87770 (CR2) and Orgueil (CI1). Unlike the well-known ee observed for amino acids in meteorites, we found that branched free and IOM-derived MCAs with 5–8 carbon atoms are essentially racemic (Aponte et al., 2014).
Hopanoids, bacterial surrogates of eukaryotic membrane sterols, are some of Earth’s most abundant natural products. Their molecular fossils are preserved in sediments as old as 1.64 billion years. On the other hand, hopanoid metabolism and function in bacteria are very poorly understood. We collaborated on an investigation of hopanoid biosynthesis in Burkholderia sp., which are opportunistic pathogens that also occupy diverse ecological niches. B. cenocepacia was investigated using deletion mutagenesis and structural characterization of the hopanoids produced. The enzymes encoded by hpnH and hpnG were essential for production of all C35 extended hopanoids which are thought to be the main physiologically active forms. Deletion of hpnI resulted in bacteriohopanetetrol (BHT) production, while ΔhpnJ produced only BHT glucosamine. Thus, HpnI is required for BHT glucosamine production while HpnJ is responsible for its conversion to the cyclitol ether. The ΔhpnH and ΔhpnG mutants could not grow under any stress condition tested, whereas ΔhpnI, ΔhpnJ and ΔhpnK displayed wild-type growth rates when exposed to detergent, but varying levels of sensitivity to low pH and polymyxin B. This study elucidated the biosynthetic pathway of hopanoids in B. cenocepacia and, further, suggests a key biosynthetic role for the conserved proteins HpnI, HpnJ and HpnK in other hopanoid-producing bacteria (Schmerk et al., 2014).
Many heterotrophic bacteria are known to form and release vesicles facilitating interactions between cells and their environment. However, vesicle production has not been described in photoautotrophs. We collaborated in a study of Prochlorococcus which is the numerically dominant marine cyanobacterium and found that they continuously release lipid vesicles containing proteins, DNA, and RNA. Vesicles carrying DNA from diverse bacteria are abundant in coastal and open-ocean seawater samples. Prochlorococcus vesicles can support the growth of heterotrophic bacterial cultures, which implicates these structures in marine carbon flux. The ability of vesicles to deliver diverse compounds in discrete packages adds another layer of complexity to the flow of information, energy, and biomolecules in marine microbial communities (Biller et al., 2014).
In earlier studies, conducted in collaboration with members of the ASU team, we examined the molecular signatures of streamer biofilm communities (SBC) that are often observed within chemosynthetic zones of Yellowstone hot spring outflow channels, where temperatures exceed those conducive to photosynthesis. Close to the hydrothermal source (75-88°C) SBC comprise thermophilic Archaea and Bacteria, often mixed communities of Desulfurococcales and uncultured Crenarchaeota, as well as Aquificae, Thermus and other organisms, each carrying a diagnostic membrane lipid biomarker signature. We then sought to evaluate their mode of carbon assimilation metabolism using various 13C-labeled substrates (bicarbonate, formate, acetate and glucose) and analyses of diagnostic membrane lipids to determine the relative uptake of these different carbon sources at two alkaline hot springs in the Lower Geyser Basin of Yellowstone National Park: Octopus Spring (UTM, Zone 12: latitude 0516053, longitude 4931215) and ‘Bison Pool’ (UTM, Zone 12: latitude 0510717, longitude 4935158). Incorporation of 13C-formate occurred only at very low rates at ‘Bison Pool’ and was almost undetectable at Octopus Spring, suggesting that formate is not an important carbon source for SBC. 13C-glucose uptake was almost undetectable. 13C-bicarbonate uptake, signifying the presence of autotrophic communities was only significant at ‘Bison Pool’ and was observed predominantly in non-specific C16-C22 fatty acids. Highest 13C uptake, at both sites, was from acetate into archaeal ether lipids and into almost all bacterial fatty acids, particularly into methyl-branched C15, C17 and C19 fatty acids that are diagnostic for Thermus/Meiothermus and some Firmicutes as well as into universally common C16:0 and C18:0 fatty acids. It is probable that these communities are energy-limited and predominantly nurtured by input of exogenous organic material from the surrounding meadows, with only a small fraction being sustained by energy-intensive autotrophic growth.
Publications
-
Aponte, J. C., Tarozo, R., Alexandre, M. R., Alexander, C. M. O. D., Charnley, S. B., Hallmann, C., … Huang, Y. (2014). Chirality of meteoritic free and IOM-derived monocarboxylic acids and implications for prebiotic organic synthesis. Geochimica et Cosmochimica Acta, 131, 1–12. doi:10.1016/j.gca.2014.01.035
-
Biller, S. J., Schubotz, F., Roggensack, S. E., Thompson, A. W., Summons, R. E., & Chisholm, S. W. (2014). Bacterial Vesicles in Marine Ecosystems. Science, 343(6167), 183–186. doi:10.1126/science.1243457
-
Briggs, D. E. G., & Summons, R. E. (2014). Ancient biomolecules: Their origins, fossilization, and role in revealing the history of life. BioEssays, 36(5), 482–490. doi:10.1002/bies.201400010
-
French, K. L., Rocher, D., Zumberge, J. E., & Summons, R. E. (2015). Assessing the distribution of sedimentary C 40 carotenoids through time. Geobiology, 13(2), 139–151. doi:10.1111/gbi.12126
-
French, K. L., Sepúlveda, J., Trabucho-Alexandre, J., Gröcke, D. R., & Summons, R. E. (2014). Organic geochemistry of the early Toarcian oceanic anoxic event in Hawsker Bottoms, Yorkshire, England. Earth and Planetary Science Letters, 390, 116–127. doi:10.1016/j.epsl.2013.12.033
-
Schmerk, C. L., Welander, P. V., Hamad, M. A., Bain, K. L., Bernards, M. A., Summons, R. E., & Valvano, M. A. (2014). Elucidation of the B urkholderia cenocepacia hopanoid biosynthesis pathway uncovers functions for conserved proteins in hopanoid-producing bacteria. Environmental Microbiology, 17(3), 735–750. doi:10.1111/1462-2920.12509
-
Schubotz, F., Hays, L. E., Meyer-Dombard, D. A. R., Gillespie, A., Shock, E. L., & Summons, R. E. (2015). Stable isotope labeling confirms mixotrophic nature of streamer biofilm communities at alkaline hot springs. Frontiers in Microbiology, 6. doi:10.3389/fmicb.2015.00042
-
Summons, R. E., Sessions, A. L., Allwood, A. C., Barton, H. A., Beaty, D. W., Blakkolb, B., … Steele, A. (2014). Planning Considerations Related to the Organic Contamination of Martian Samples and Implications for the Mars 2020 Rover. Astrobiology, 14(12), 969–1027. doi:10.1089/ast.2014.1244
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Roger Summons
Project Investigator
Jose Aponte
Co-Investigator
Steven Biller
Co-Investigator
Katherine French
Co-Investigator
Alex Kasprak
Co-Investigator
D'Arcy Meyer-Dombard
Co-Investigator
Dan Rothman
Co-Investigator
Florence Schubotz
Co-Investigator
Julio Sepulveda
Co-Investigator
Everett Shock
Co-Investigator
Miguel Valvano
Co-Investigator
Paula Welander
Co-Investigator
Jessica Whiteside
Co-Investigator
Kenneth Williford
Co-Investigator
-
RELATED OBJECTIVES:
Objective 4.1
Earth's early biosphere.
Objective 4.2
Production of complex life.
Objective 5.1
Environment-dependent, molecular evolution in microorganisms
Objective 5.2
Co-evolution of microbial communities
Objective 7.1
Biosignatures to be sought in Solar System materials