2013 Annual Science Report
 University of Hawaii, Manoa
Reporting | SEP 2012 – AUG 2013
University of Hawaii, Manoa
Reporting | SEP 2012 – AUG 2013
Ice Chemistry: Radiation Induced Chemical Processing
Project Summary
Prebiotic molecules such as amino acids, sugar, and sugar alcohols are thought to be delivered to the early Earth by meteorites and comets and may have played crucial role in the origin of life. However, there is no conclusive evidence of these molecules found in the interstellar medium. However, simple precursors such as formaldehyde, acetaldehyde, acetone, propanal, propenal, and acetic acid were observed in the interstellar medium such as toward the star forming region SgrB2. Extraterrestrial ices with abundant molecules like water, methane, carbon monoxide, carbon dioxide, ammonia, and methanol are exposed to ionizing radiation such as galactic cosmic radiation and UV radiation. Here, we have been investigating the effect of ionizing radiation on simple astrophysical ice representatives in the solid state using FTIR, UV-VIS, Raman spectroscopy as well as the products analyzed in the gas phase (fragment free reflectron time-of-flight mass spectroscopy combined with single photon ionization (ReTOF-PI)) while subliming to the gas phase after a controlled temperature desorption. Our laboratory simulation experiments provide clear evidence of the formation of large number of aldehydes, ketones such as acetaldehyde, acetone in methane-carbon monoxide ices, formation of simple sugar alcohols (glycerol) in methanol ices and possibly formation of amino acid (glycine) in mixed ices of water, carbon dioxide, methane, and ammonia.
Project Progress
Enceladus Frozen Surface (with Dr. S. Pilling and Alexandre B. Souza)
Enceladus, one of Saturn’s moons, is a frozen body covered by ice and snow. The surface has temperatures from 40 K to 160 K, and its composition is basically made of water, carbon dioxide, methane, and ammonia as recently discovered by NASAs Cassini probe. The molecules on Enceladus surface are continuously processed by ionizing radiation, such as VUV and X-ray photons, energetic electrons and possible, cosmic rays. This may induce chemical changes and lead to the formation of complex molecules, some of them with very important from the astrology point of view. Ice mixtures of water, carbon dioxide, methane, and ammonia were processed by ionizing radiation, such as energetic electrons and continuous VUV photons. The radiation induced chemical processing was probed via complementary methods (infrared spectroscopy, Raman spectroscopy, UV-VIS spectroscopy) and in the gas phase via mass spectroscopy (Quadrupole Mass Spectrometry and Reflectron Time-of-Flight Mass Spectrometry). A preliminary data analysis suggests the formation of glycine (NH2CH2COOH) in the processed ices. It is expected that this research clarifies crucial chemical processes involving prebiotic molecules on this moon, placing important pieces in the puzzle of the origin of life in the Solar System.
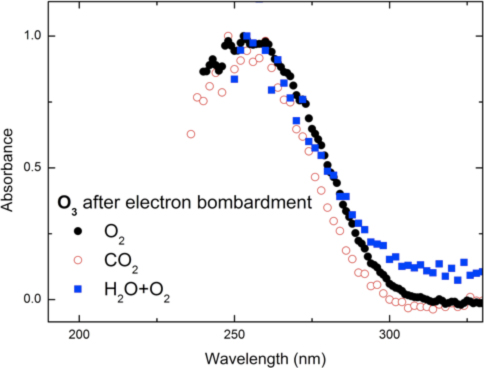
Ozone (O3) on the Surface of Ganymede (with Giovanni Strazzulla)
Ozone (O3) has been detected on the surface of Ganymede (moon of Jupiter) via observation of the Hartley band through the use of ultraviolet spectroscopy and it is largely agreed that this is formed by radiolytic processing via interaction of magnetospheric energetic ions and/or electrons with oxygen-bearing ices on Ganymede’s surface. Interestingly, a clearly distinct band near 300 nm within the shoulder of the UV-VIS spectrum of Ganymede was also observed, but currently lacks an acceptable physical or chemical explanation. Consequently, the primary motivation behind this work was the collection of UV-Vis absorption spectroscopy of ozone formation by energetic electron bombardment of a variety of oxygen-bearing ices (oxygen, carbon dioxide, water) relevant to this moon as well as other Solar System and interstellar icy bodies. Ozone was indeed synthesized in pure ices of molecular oxygen, carbon dioxide and a mixture of water and oxygen, in agreement with previous studies (Fig. 1). The Hartley band of the ozone synthesized in these ice mixtures was observed in the UV-VIS spectra and compared with the spectrum of Ganymede. In addition to, a solid state ozone absorption cross section of 6 × 10-17 cm2 molecule-1 was obtained from the UV-Vis spectral data. Ozone was not detected in a mixture of water and carbon dioxide upon exposure to ionizing radiation. The UV-VIS absorption spectrum of irradiated water – carbon dioxide mixture did not yield any distinct absorption features near the unidentified shoulder of Ganymede’s spectra, however a spectrally “red” UV continuum is observed and appears to reproduce well what is observed in a large number of icy moons such as Europa. Our studies indicate that energetic processing of mixtures of carbon and water bearing ices can explain the appearance of the feature. In addition we demonstrate that if patches of pure, segregated, carbon dioxide exist on those objects then molecular oxygen as formed upon electron bombardment can be trapped and sublimate with the bulk of carbon dioxide thus contributing to the tenuous atmospheres observed in icy moons like Callisto, Dione and Rhea.
Formation of Carbonyl-Bearing Molecules in Extraterrestrial Ices (with Brant Jones, Aleca Borsuc and Ralf Kaiser)
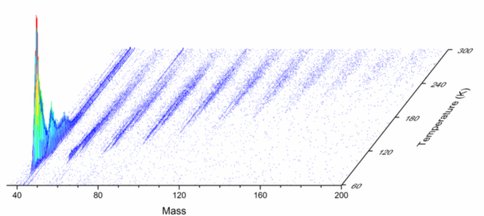
Interstellar grains covered with an icy ‘mantle’ consisting mainly water (H2O) followed by methanol (CH3OH), carbon monoxide (CO), carbon dioxide (CO2), methane (CH4) ammonia (NH3), and formaldehyde (H2CO) with a thicknesses of up to a few hundred nanometers. The interaction of these ices with galactic cosmic rays and with the internal ultraviolet field inside cold molecular clouds has been demonstrated for last few decades to chemically modify the pristine ices via non-thermal, non-equilibrium chemistry involving supra-thermal reactants such as hydrogen, oxygen, nitrogen, and carbon atoms. Therefore, in cold molecular clouds, an interaction of ionizing radiation with ice-coated nanoparticles is expected to lead to the synthesis of complex organic molecules such as aldehydes, ketones, carboxylic acids, esters that are considered as precursor of prebiotic molecules. In order to investigate the formation of these carbonyl-bearing molecules in extraterrestrial ices, ice mixtures of methane and carbon monoxide were exposed to ionizing radiation in form of energetic electrons. The radiation induced chemical processing of the mixed ices along with isotopically labeled counterparts were probed using on-line and in-situ via infrared spectroscopy (solid state) aided with reflectron time-of-flight mass spectrometry (ReTOF) coupled to single photon photoionization at 10.49 eV (gas phase). Upon sublimation of the newly synthesized molecules, ReTOF together with isotopic shifts of the mass-to-charge ratios was exploited to identify eleven product classes containing molecules with up to six carbon atoms, which can be formally derived from C1-C5 hydrocarbons incorporating up to three carbon monoxide building blocks (Fig. 2). The classes are (i) saturated aldehydes/ketones, (ii) unsaturated aldehydes/ketones, (iii) doubly unsaturated aldehydes/ketones, (iv) saturated dicarbonyls (aldehydes/ketones) (v) unsaturated dicarbonyls (alde-hydes/ketones), (vi) saturated tricarbonyls (aldehydes/ketones), molecules containing (vii) one carbonyl – one alcohol (viii) two carbonyls – one alcohol, (ix) one carbonyl – two alcohol groups along with (x) alcohols and (xi) diols. Our studies provide clear evidence that among these observed molecules, acetaldehyde (CH3CHO) and acetone (CH3COCH3) and possibly propanal (HCOC2H5) together with propenal (HCOC2H3) can be formed upon interaction of methane – carbon monoxide ices with energetic electrons.
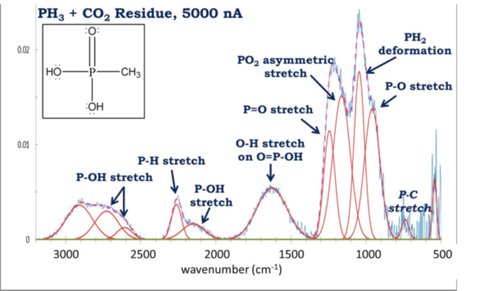
Formation of alkyl phosphonic acids using phosphine ices (with Andrew Turner and Ralf Kaiser)
The low solubility of phosphate minerals make them an unlikely source of phosphorus for the first organisms, and extraterrestrial phosphorus-bearing compounds, such as those formed from hydration of phosphide minerals found in meteorites, have been proposed as alternative sources. The only organic phosphorus compounds on the Murchison meteorite were alkyl phosphonic acids. Previous studies have shown that the simplest C1 and C2 alkyl phosphonic acids could be formed from irradiated mixtures of organic compounds with aqueous phosphite, which is a product of phosphide hydrolysis. The recent discovery of phosphine (PH3) in the interstellar medium expands the possible origins of alkyl phosphonic acids to formation on interstellar icy grains. This research simulates conditions in molecular clouds such as low temperatures, ultrahigh vacuum pressures, the chemical composition, and ionizing energy sources in order to unravel the formation mechanisms of alkyl phosphonic acids in phosphine-bearing ices (Fig. 3).
Experimental Studies on the Formation of D2O (with Chris Bennett and Ralf Kaiser)
The recent detection molecular oxygen (O2) toward ρ Oph A suggests that the gas phase abundance accounts for only ~1 % of the total interstellar oxygen budget indicating that most molecular oxygen could be condensed depleted on interstellar grains in cold molecular clouds. On the other hand, water ices have been commonly identified on interstellar grains at abundances up to three orders of magnitude higher than predicted from simple gas-phase freeze-out mechanisms. Considering that the observed abundance of molecular oxygen is lower, but water higher than expected from simple gas phase condensation models, molecular oxygen might have been converted to water on interstellar grains. Here we demonstrate via laboratory experiments that water – in the form of D2-water – can be easily generated in oxygen ices at temperatures as low as 10 K upon interaction with implanted deuterium ions (Fig. 4). We also present detailed mechanistical studies and elucidate possible reaction pathways for the formation of D2-water. These studies are important to rationalize the conversion of molecular oxygen into water ices in interstellar grains.
Acoustic Levitation of Micrometer Sized Particles (with Stephen Brotton and Ralf Kaiser)
An original apparatus comprising of an acoustic levitator enclosed within a pressure compatible process chamber was designed and constructed (Fig. 5). To characterize any chemical and physical modifications of the levitated particle, the chamber is interfaced to complimentary, high-sensitivity Raman (4390 – 170 cm−1), and Fourier transform infrared (FTIR) (10,000 – 500 cm−1) spectroscopic probes. The temperature of the levitated particle can be accurately controlled by heating using a carbon dioxide laser emitting at 10.6 μm. The advantages of levitating a small particle combined with the two spectroscopic probes, process chamber, and infrared laser heating makes novel experiments possible relevant to the fields of, for example, planetary science, astrobiology, and combustion chemistry. We demonstrate that this apparatus is well suited to study the dehydration of a variety of particles including minerals and biological samples; and offers the possibility of investigating combustion processes involving micrometer-sized particles such as graphite. Furthermore, we show that the FTIR spectrometer enables the study of chemical reactions on the surfaces of porous samples and scientifically and technologically relevant, micrometer-thick levitated sheets. The FTIR spectrometer can also be used to investigate non-resonant and resonant scattering from small, irregularly-shaped particles across the midinfrared range from 2.5 μm to 25 μm, which is relevant to scattering from interplanetary dust and biological, micrometer-sized samples but cannot be accurately modeled using Mie theory. As a proof of concept study, we observed the variations in the Raman spectra as micrometer-sized gypsum (CaSO4 • 2 H2O) and epsomite crystals (MgSO4 • 7 H2O) were dehydrated in anhydrous nitrogen gas at distinct temperatures yielding ultimately their anhydrates.
Publications
-
Bennett, C. J., Brotton, S. J., Jones, B. M., Misra, A. K., Sharma, S. K., & Kaiser, R. I. (2013). High-Sensitivity Raman Spectrometer To Study Pristine and Irradiated Interstellar Ice Analogs. Anal. Chem., 85(12), 5659–5665. doi:10.1021/ac303259y
-
Bennett, C. J., Ennis, C. P., & Kaiser, R. I. (2014). EXPERIMENTAL STUDIES ON THE FORMATION OF D 2 O AND D 2 O 2 BY IMPLANTATION OF ENERGETIC D + IONS INTO OXYGEN ICES. The Astrophysical Journal, 782(2), 63. doi:10.1088/0004-637x/782/2/63
-
Brotton, S. J., & Kaiser, R. I. (2013). In Situ Raman Spectroscopic Study of Gypsum (CaSO 4 ·2H 2 O) and Epsomite (MgSO 4 ·7H 2 O) Dehydration Utilizing an Ultrasonic Levitator. The Journal of Physical Chemistry Letters, 4(4), 669–673. doi:10.1021/jz301861a
-
Brotton, S. J., & Kaiser, R. I. (2013). Novel high-temperature and pressure-compatible ultrasonic levitator apparatus coupled to Raman and Fourier transform infrared spectrometers. Review of Scientific Instruments, 84(5), 055114. doi:10.1063/1.4804647
-
Jones, B. M., & Kaiser, R. I. (2013). Application of Reflectron Time-of-Flight Mass Spectroscopy in the Analysis of Astrophysically Relevant Ices Exposed to Ionization Radiation: Methane (CH 4 ) and D4-Methane (CD 4 ) as a Case Study. The Journal of Physical Chemistry Letters, 4(11), 1965–1971. doi:10.1021/jz400692r
-
Jones, B. M., Kaiser, R. I., & Strazzulla, G. (2014). UV-VIS, INFRARED, AND MASS SPECTROSCOPY OF ELECTRON IRRADIATED FROZEN OXYGEN AND CARBON DIOXIDE MIXTURES WITH WATER. The Astrophysical Journal, 781(2), 85. doi:10.1088/0004-637x/781/2/85
-
Kaiser, R. I., Maity, S., & Jones, B. M. (2014). Infrared and reflectron time-of-flight mass spectroscopic analysis of methane (CH 4 )–carbon monoxide (CO) ices exposed to ionization radiation – toward the formation of carbonyl-bearing molecules in extraterrestrial ices. Phys. Chem. Chem. Phys., 16(8), 3399–3424. doi:10.1039/c3cp54255f
-
Kaiser, R. I., Stockton, A. M., Kim, Y. S., Jensen, E. C., & Mathies, R. A. (2013). ON THE FORMATION OF DIPEPTIDES IN INTERSTELLAR MODEL ICES. The Astrophysical Journal, 765(2), 111. doi:10.1088/0004-637x/765/2/111
-
Kim, Y. S., & Kaiser, R. I. (2012). ELECTRON IRRADIATION OF KUIPER BELT SURFACE ICES: TERNARY N 2 -CH 4 -CO MIXTURES AS A CASE STUDY. The Astrophysical Journal, 758(1), 37. doi:10.1088/0004-637x/758/1/37
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Ralf Kaiser
Project Investigator
Brant Jones
Co-Investigator
Aleca Borsuk
Collaborator
S. Pilling
Collaborator
Alexandre Souza
Collaborator
Giovanni Strazzulla
Collaborator
Andrew Turner
Collaborator
-
RELATED OBJECTIVES:
Objective 3.1
Sources of prebiotic materials and catalysts
Objective 3.2
Origins and evolution of functional biomolecules